Multiple Myeloma
Latest News
Latest Videos

CME Content
More News

Elranatamab is an investigational B-cell maturation antigen CD3-targeted bispecific antibody that may become the next standard of care for multiple myeloma, according to researchers.
Total cost of care for multiple myeloma can be significantly affected by selection of dosing regimen.

Key opinion leaders emphasize the critical role of effective clinical pathways for treatment in multiple myeloma.
Because of CRS and neurologic toxicity risk, the therapy is available through a REMS program.
Idecabtagene vicleucel prolonged progression-free survival by more than 1 year in a difficult-to-treat patient population with relapsed or refractory multiple myeloma.

4-drug treatment regimens could provide optimal care strategies for patients with multiple myeloma.

Experts discuss the results of the IKEMA and CANDOR trials.

Isatuximab and daratumumab offer promising treatment options for previously treated multiple myeloma.

Dr Haumschild leads a discussion surrounding key considerations when using anti-CD38 drugs.

Medical experts emphasize anti-CD38 agent utilization for treatment of patients with multiple myeloma.

The trial is the first randomized phase 3 study evaluating the efficacy and safety of ciltacabtagene autoleucel patients with relapsed and lenalidomide-refractory multiple myeloma.

A panel of experts navigate the treatment landscape in multiple myeloma.

Kathryn Maples, PharmD, BCOP, provides insights regarding unmet needs in treatment for relapsed/refractory multiple myeloma.

A panel of experts explore burdens facing patients diagnosed with RRMM.

Ryan Haumschild, PharmD, MS, MBA, leads a panel surrounding individualized strategies for treating patients with relapse/refractory (RR) MM.

Many patients have baseline risk factors present at diagnosis because of older age and disease-related elements.
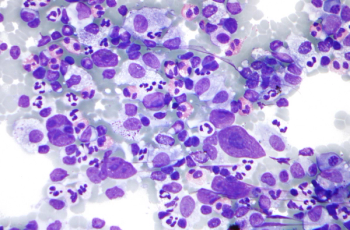
A better understanding of the pathogenesis of monoclonal gammopathy of clinical significance would help guide clinicians treating the complex condition.

Ryan Haumschild, PharmD, MS, MBA, leads a discussion surrounding relapsed/refractory multiple myeloma (MM).

Expert discusses the patient-reported outcomes from the GRIFFIN trial at the final study analysis after all patients completed 1 year of follow-up post maintenance therapy.

Researchers also noted an increasing rate of seroconversion with COVID-19 booster vaccination series.

Research Continues Evaluating Belantamab Mafodotin in Relapsed/Refractory Multiple Myeloma
Belantamab mafodotin-blmf is a first-in-class, anti-B-cell maturation antigen therapy indicated for adults with relapsed or refractory multiple myeloma who have received at least 4 prior therapies.

Elranatamab is a B-cell maturation antigen (BCMA)-CD3-targeted bispecific antibody.

In the inpatient acute care setting, oncology pharmacists maximize return on investments of oncology products, produce optimal outcomes, and ensure that quality of care is consistent and safe.

By 2019, lung cancer mortality rates decreased by 5.4% in males since 2015, while deaths from ovary cancer have also declined rapidly over the past 2 decades.
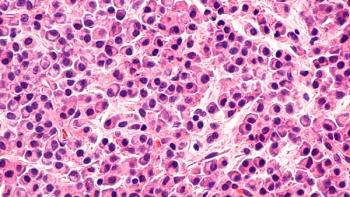
The indication is approved under accelerated approval based on response rate, and continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.