Top news of the day from across the health care landscape.

Top news of the day from across the health care landscape.

Patients with psoriasis reported frequent use of complementary and alternative treatments when their initial therapies did not help or had adverse effects.

The study focused on the comparative risk of infection across 7 systemic biologics.

Top news of the day from across the health care landscape.
Top news of the day from across the health care landscape.
A head-to-head trial evaluated quality of life improvements in patients with moderate-to-severe psoriasis treated with secukinumab (Cosentyx) or ustekinumab (Stelara).

Top news of the week from Specialty Pharmacy Times.

With this approval, dupilumab (Dupixent) offers a biologic therapy option for patients 12 to 17 years of age who have moderate-to-severe atopic dermatitis.

Approximately 1.7 million insured US patients are burdened with moderate to severe plaque psoriasis.

Tildrakizumab was approved by the FDA as a subcutaneous therapy for patients with moderate-to-severe psoriasis.
Analysis of care in a routine clinical practice setting show that 40-week treatment of biologic dupilumab (Dupixent, Sanofi-Regeneron) plus noncosmetic topical moisturizer (emollient) is associated with significantly improved symptoms and health-related quality of life (HRQoL) in particular patients with atopic dermatitis (AD).

Data from an analysis of multiple phase 3 studies highlight improvements in mobility, self-care, and usual activities for patients with moderate-to-severe psoriasis taking secukinumab (Cosentyx).
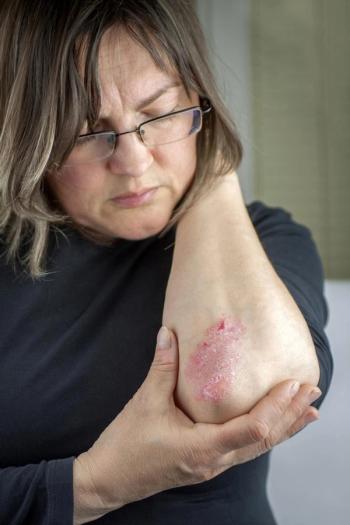
The FDA has approved Ilumya (tildrakizum- ab-asmn) from Sun Pharmaceutical Industries Ltd to treat moderate to severe plaque psoriasis.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.
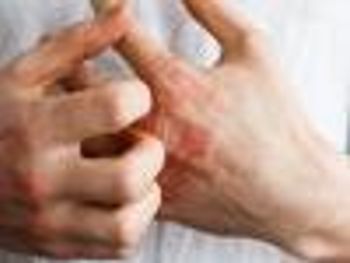
Data from 2 phase 3 trials showed significant improvement in disease activity in patients with moderate-to-severe atopic dermatitis treated with baricitinib (Olumiant).

A study of methotrexate in patients with psoriasis found that the immunosuppressive drug was more effective in patients without psoriatic arthritis than those with the added condition.
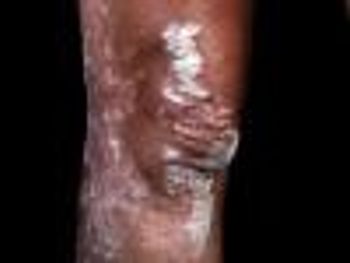
Patients administered guselkumab achieved an improvement of 90% or more Psoriasis Area Severity Index score than those administered secukinumab.

A new study shows high prevalence of atopic dermatitis among adults, illustrating the need for more available treatment options.
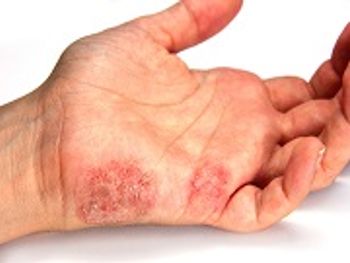
Officials with the FDA have granted Priority Review to dupilumab (Dupixent, Sanofi) for moderate-to-severe atopic dermatitis in adolescent patients aged 12 to 17 years.

If approved for this indication, dupilumab (Dupixent) will be the first systemic biologic medicine to treat adolescents with moderate-to-severe atopic dermatitis.
Sun Pharma announced the availability of its treatment for moderate-to-severe plaque psoriasis in the United States.
Tildrakizumab-asmn (Ilumya) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults.

Tildrakizumab-asmn (Ilumya), an injectable interleukin-23 (IL-23) inhibitor, was approved by the FDA in March for adults who are candidates for systemic therapy or phototherapy.

Top news of the day from across the health care landscape.

Phase 3 trial results for dupilumab (Dupixent) demonstrate improvement in skin clearing, itch, and certain quality of life measurements for adolescents with atopic dermatitis.