
The FDA linked cerebral folate deficiency to developmental delays with autistic features, although research remains ongoing.

The FDA linked cerebral folate deficiency to developmental delays with autistic features, although research remains ongoing.

The indication is for the treatment of adult and pediatric patients who weigh at least 30 kg.

Guselkumab becomes the first and only IL-23 inhibitor with a fully subcutaneous induction regimen for adults with ulcerative colitis.
Participating in a pharmacist-led secondary intervention clinic helped achieve large reductions in cholesterol levels among patients who have experienced an acute coronary syndrome.
Bumetanide nasal spray provides patients with edema an alternative to burdensome oral or intravenous diuretic options.
A series of PCSK9 inhibitors were found to be tolerable and effective at reducing low-density lipoprotein cholesterol in solid organ transplant recipients.
Investigators from the phase 3 COAST 1 trial demonstrated the efficacy and safety of amlitelimab in patients with moderate-to-severe atopic dermatitis.
Experts gathered to discuss the critical need for real-time learning, multidisciplinary collaboration, and comprehensive patient and family support to address unforeseen complications in gene therapy.
Speakers at an ASTCT Gene Therapy Summit highlighted novel conditioning approaches without the use of busulfan, which can cause toxic effects in patients with hematologic malignancies.
Experts at the ASTCT Gene Therapy Summit discussed the multifaceted nature of the gene therapy process for patients with sickle cell disease.
Both cemdisiran alone and combined with pozelimab improved the Myasthenia Gravis Activities of Daily Living score in patients with generalized myasthenia gravis.
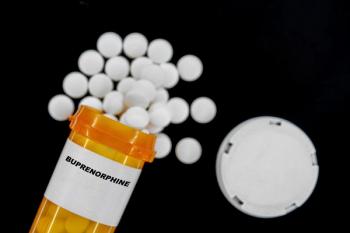
Pharmacies increasingly dispense buprenorphine, enhancing access to opioid addiction treatment while embracing AI and telepharmacy innovations for better care.
Results from the phase 3 SAPPHIRE trial show significant improvements in motor function in children and adults with spinal muscular atrophy.
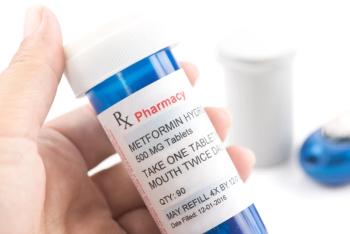
If long-term efficacy results are positive, metformin can become an accessible, affordable treatment option for autosomal dominant polycystic kidney disease (ADPKD).
A systematic review and meta-analysis revealed key insights into lipid parameters in patients with systemic sclerosis.

State policymakers should target bad actors while protecting access.
High-density lipoprotein cholesterol, known for protective cardiovascular effects, could play a role in reducing the risk of stress urinary incontinence.

The complete response letter (CRL) stated that vatiquinone's new drug application (NDA) did not provide substantial evidence of efficacy and would need to be evaluated in a new trial.
Unicycive Therapeutics secures a US patent for UNI-494, a promising treatment for chronic kidney disease, enhancing its development and partnership potential.
By improving low-density lipoprotein cholesterol (LDL-C) levels, statins reduced complications after pipeline embolization device implantation for intracranial aneurysms.

New research reveals that novel biomarkers enhance risk prediction for kidney failure and mortality in chronic kidney disease (CKD), paving the way for personalized treatment.

Amy C. Nieto delves into the latest advancements in non-small cell lung cancer (NSCLC) treatment, including sunvozertinib, a newly-approved therapy for challenging EGFR exon 20 insertion mutations.
Multiple FDA-approved drugs without lipid-lowering indications were identified to have potential lipid-lowering properties.

The researchers speculate that this is because of the antioxidant and anti-inflammatory properties of coffee and tea.

Designed for use in Celiac disease, larazotide was found to hasten resolution of multisystem inflammatory syndrome symptoms in children with the condition.

Brad Cochran of Cardinal Health explains the One Voice Initiative, which aims to bolster independent pharmacies.
Mona El-Mouwfi explores how pharmacists enhance hydroxyurea therapy for sickle cell disease, improving patient outcomes and addressing treatment barriers effectively.

Intravenous immunoglobulin (IVIG) is a vital treatment for immunodeficiency and autoimmune disorders, with diverse mechanisms, brand-specific differences, and patient-centered considerations shaping safe and effective use.

The approval builds upon concizumab’s previous December 2024 clearance and allows for treatment in patients with hemophilia A or B, both with or without inhibitors.
Ivermectin shows promise in reducing malaria cases, enhancing vector control, and highlighting pharmacists' vital roles in public health strategies.