
BLU-667 is being investigated for the treatment of cancers caused by an alteration in the receptor tyrosine kinase.

BLU-667 is being investigated for the treatment of cancers caused by an alteration in the receptor tyrosine kinase.

Half of all HAE drug expenses were billed through the pharmacy benefit and half through the medical benefit.
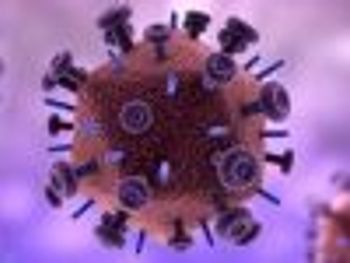
HIV targets and disables a pathway involved in blocking viral activity and clearing infection.

At the 2018 National Community Oncology Dispensing Association (NCODA) Spring Summit, Ali McBride, PharmD, MS, BCPS, BCOP, explains what strategies can help ensure patients are adherent to oral chemotherapy regimens.

Poor study design and reporting in animal research compromises the ethical review of proposed human drug trials.

A synthetic bridged nucleic acid was found to greatly improve the accuracy of gene-editing technology, which could potentially lead to new therapies for genetic diseases.

People living with HIV are at elevated risk for hyponatremia, the most common electrolyte disorder seen in hospitals.

The new method indexes thousands of cells simultaneously and could potentially improve understanding of various diseases at a molecular level.

The findings suggest that a patient’s diet may impact their ability to combat head and neck cancer.

At the 2018 National Community Oncology Dispensing Association (NCODA) Spring Summit, Ray Bailey, BPharm, RPh, Pharmacy Director at Florida Cancer Speciality, discusses interventions aimed at reducing the waste and cost of expensive oral chemotherapy treatments.

In the largest study of its kind, breast cancer survivors were not found to have a greater mortality risk due to heart disease than the general population.

Unintentional weight loss may allow physicians to detect cancer sooner.

Burosumab (Crysvita, Ultragenyx) is the first treatment approved for x-linked hypophosphatemia in adults and children ages 1 and older.

Brandon Newman, PharmD, CSP, Program Director, Vanderbilt Specialty Pharmacy, speaks about how specialty pharmacies have to adjust their operations to meet the needs of their patients.

Following the approval of voretigene neparvovec, new treatments are on the horizon for retinitis pigmentosa.

When deciding to include a pharmacy in a distribution network, a manufacturer will look for factors that positively differentiate the pharmacy, as well as factors that will likely help the target patient population.

Top news of the week from Specialty Pharmacy Times.
Several barriers to value-based care exist, including finances and cost, meaning both the financial investments required by small- and mid-sized provider groups and the size of corresponding incentives.
Patients with severe hemophilia are at the highest risk for spontaneous bleeds, therefore are associated with the highest costs.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.
Spending on specialty medications is rapidly approaching half of overall medicine spending from a small volume of prescriptions filled.

Pharmacy benefit manager reports specialty drugs drive 97% of spending for hereditary angioedema care.

Top news of the day from across the health care landscape.

Top news of the day from across the health care landscape.
Thirty two million Americans are affected by migraines, and women are affected 3 times more than men.

Managed care organizations and pharmacy benefit managers are increasingly using strict approaches to manage drugs, including formulary exclusions.

At the 2018 National Community Oncology Dispensing Association (NCODA) Spring Summit, Howard Cohen, RPh, MS, FASHP, from Smilow Cancer Hospital, Yale-New Haven Health, discusses how information technology can be utilized to improve medication adherence on patients with cancer.

Patients treated with erenumab had nearly 3-fold higher odds of having their migraine days cut by at least 50%.

Study finds improving adherence to disease-modifying multiple sclerosis medications could reduce clinical relapses while saving on medical costs.

A review found an elevated risk of cancer progression with increasing wait time between positive screening and start of diagnostic testing.