
Pharmacists are key to closing care gaps and supporting long-term recovery.

Pharmacists are key to closing care gaps and supporting long-term recovery.

Fast-melt tablets may help improve adherence by offering patients a convenient alternative to traditional pills, especially for those experiencing pill fatigue or managing multiple medications.

The Advisory Committee on Immunization Practices (ACIP) voted to recommend that COVID-19 vaccines be administered through individual-based decision-making.

Discover effective strategies for managing eczema, dry skin, sun protection, and acne with expert recommendations for OTC treatments.

Expert highlights how taste-masking, dose, and particle size influence patient adherence and the clinical performance of fast-dissolving delivery systems.
A multicenter registry analysis reveals that high-risk cardiovascular patients often miss opportunities to receive pneumococcal and influenza vaccinations, showcasing gaps that could be filled by pharmacists.

The BMJ study highlights that young children vaccinated against influenza in October experience the greatest protection, with lower infection rates than those vaccinated earlier.

Members of the Advisory Committee on Immunization Practices (ACIP) voted to restrict the combined measles, mumps, rubella, and varicella (MMRV) vaccine and hepatitis B vaccine for certain age groups.

With this approval, an effective, nonsteroidal treatment option is available for young patients.
Participating in a pharmacist-led secondary intervention clinic helped achieve large reductions in cholesterol levels among patients who have experienced an acute coronary syndrome.
Long-term care pharmacists are significant in lessening the vaccine hesitancy hurdle among facility residents.
Discover the benefits of fast melt tablets, designed for easy swallowing and quick disintegration, enhancing patient convenience and adherence.

Pharmacists play a critical role in optimizing triazole therapy in preventing and managing invasive fungal infections.

The extensive selection of OTC products for GI problems can be challenging for patients to navigate.
West Coast states unite to enhance vaccine access and guidance for respiratory viruses, prioritizing public health and safety ahead of the 2025-2026 season.
Anti-Amyloid Antibody Treatments, Subcutaneous Formulations, and Improved Symptom Relief Are Offering New Hope for Patients

Enrollment in the Supplemental Nutrition Assistance Program or another food assistance program could help ameliorate food insecurity that contributes to long COVID.

Additionally, the FDA granted a provisional interchangeability designation to both biosimilars.

Travelers face rising infectious disease risks, including measles, chikungunya, chagas disease, and others.
This study demonstrates the feasibility and impact of a pharmacist-led direct challenge approach to penicillin allergy delabeling in a community hospital setting.
A recent study highlights the AOV dual-chamber system as a faster, safer option for RSV vaccine reconstitution, enhancing efficiency for health care providers.
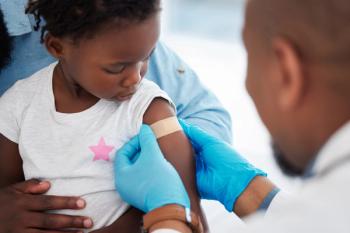
Misinformation is fueling parental fears and a concerning drop in childhood vaccines.

Engagement and recognition of pharmacist expertise grows as state psychedelic bills expand.

Breast cancer survivors face a small but significant risk of developing secondary cancers, influenced by age, treatment, and lifestyle factors.

Pharmacists face challenges as recent federal policy changes threaten their expanded roles in vaccination and public health, impacting patient access to care.

GLP-1 receptor agonists enhance glycemic control and weight management in youth with type 2 diabetes (T2D) and obesity; however, safety concerns persist.

Research reveals that this population may experience lower short-term mortality rates after major surgery, challenging traditional BMI guidelines.

Use of educational resources at a gastrointestinal office improved pneumococcal vaccine uptake in patients with celiac disease.

Crystal Hodge, PharmD, BCIDP, BCPS, discusses a 3-pronged approach—isolating, vaccinating, and educating—to help pharmacists manage respiratory virus season and routine immunizations like MMR.

Abdulwhab Shremo Msdi, PharmD, BCIDP, advances research in infectious diseases and microbiome-related conditions.