
Today, we’re celebrating Jennifer Cardona, PharmD, who works at an independent pharmacy in Puerto Rico.

Today, we’re celebrating Jennifer Cardona, PharmD, who works at an independent pharmacy in Puerto Rico.
The FDA released a statement on taking action to increase US supplies to support the response to the coronavirus disease 2019 by providing instructions to manufacturers importing personal protective equipment and other devices.

The new edge-computing platform may expand the arsenal of health surveillance tools used to forecast seasonal flu and other viral respiratory outbreaks, such as COVID-19 or severe acute respiratory syndrome.
Here is an overview of reported investigations of existing medications.

Levels of the HIV pre-exposure prophylaxis (PrEP) drug tenofovir among African adolescent girls and young women were more than 30% lower in those who were pregnant than in those who had recently given birth.

The program will award grants for training and development of educational resources for employees in high risk occupations who serve the public during emergencies and who need skills to protect their own health.
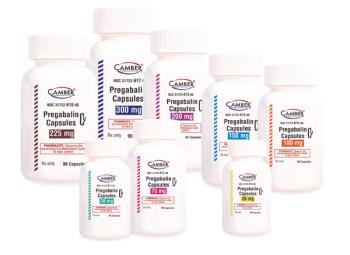
Pregabalin capsules is used to treat pain caused by nerve damage due to diabetes or shingles infection.

Pennsylvania Governor Tom Wolf’s administration has decided that in-state and out-of-state health care providers can treat patients via telemedicine during the coronavirus disease 2019 pandemic.

Officials with the FDA have said they are beginning to see unauthorized test kits marketed for consumers to test for COVID-19 at home.
Hoth Therapeutics and Voltron Therapeutics will collaborate to develop the self-assembling vaccine technology as a means to aid patients at risk of being infected with COVID-19.

The study, which includes data from asthma patient samples and mouse models, is the first to definitively show a relationship between iron build-up in the lung cells and tissues and the severity of asthma, according to the researchers.

The multi-site study of patients aged 65 years or older examined the levels of vitamin D levels in blood serum, and nutrition and its impact on mobility.

As the role of pharmacists grows into a new phase, the role of the pharmacy technician is expanding as well.

The test can be used to identify large numbers of infected patients and asymptomatic carriers, which would help support containment efforts and slow the spread of the coronavirus disease 2019 (COVID-19).

Caplyta is an oral, once daily medicine approved for the treatment of schizophrenia in adults.
Not enough people are taking seriously frequent recommendations to practice social distancing, he said.

Why is your patient using conjugated estrogens vaginal cream for nosebleeds?

The FDA has issued a new guidance to sponsors and health care providers regarding certain Risk Evaluation and Mitigation Strategy-required testing during the coronavirus disease 2019 pandemic.

The COVID-19 High Performance Computing Consortium is a partnership of federal government, tech industry, and academic leaders whose goal is to stop the virus.
A study of the second HIV patient to undergo successful stem cell transplantation from donors with an HIV-resistant gene found that there was no active viral infection in the patient’s blood 30 months after they stopped antiretroviral therapy.
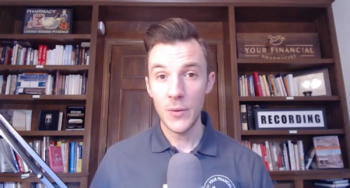
Tim Ulbrich, PharmD, co-founder and CEO of Your Financial Pharmacist, explains how your finances may be affected by the coronavirus and how your can reinforce your financial situation.
The US Pharmacopeia (USP) is providing free technical assistance to developers of antiviral drugs and vaccines that will support the public health response to the coronavirus disease 2019 (COVID-19) pandemic.

Pharmacy Moms Group special task force develops guidelines as precautions are implemented amid the COVID-19 pandemic.

A new commentary suggests that public health leaders should guide world leaders through the coronavirus disease 2019 pandemic.

A report published noted that there are subpopulations of pediatric patients with COVID-19 who have an increased risk for more significant illness.

Steven C. Hoffart, PharmD, owner and operator of Magnolia Pharmacy in Magnolia, Texas, said his pharmacy had run out of prepared hand sanitizer when he saw a need for many people in his area.

The 12 groups represent practice settings of nearly all pharmacists in the United States.
“We’re pretty devastated,” said Jessica Harris, a friend of Rutter, in an interview with WFSB. “She beat cancer and lost the battle to coronavirus—it’s just crazy.”

More than 90% of physicians, nurses, and pharmacists are concerned about COVID-19 affecting their patients, whereas 68% of doctors and 67% of nurses feel they are reasonably prepared for a COVID-19 outbreak in their area.