Based on real-world outcomes, there is an unmet need for an effective therapy to be used among patients aged 75 years or older with relapsed/refractory diffuse large B-cell lymphoma.

Based on real-world outcomes, there is an unmet need for an effective therapy to be used among patients aged 75 years or older with relapsed/refractory diffuse large B-cell lymphoma.

Regular physical activity helps to facilitate a healthy gut microbiome and lowers inflammation, which were observed in patients independent of body mass index.
Long-term data showed no difference in the clinical benefit between the treatments for HER2-negative early breast cancer with homologous recombination deficiency.

Study findings may make durvalumab plus chemotherapy a new standard of care option for patients with advanced biliary tract cancer.
Expert discusses clinical outcomes of the phase 3 ZUMA-7 trial assessing axicabtagene ciloleucel versus standard-of-care in second-line large B-cell lymphoma by metabolic tumor volume.

At 3 and 6 months, rivaroxaban was found to be as effective and safe as apixaban in the composite outcome of recurrent venous thromboembolism or bleeding-related hospitalization.

The phase 3 clinical trial shows that the 5-year OS for patients with HR+/HER2- advanced breast cancer in the abemaciclib plus fulvestrant group was 41.2% vs 29.2% for the placebo arm.
Results from the phase 3 CAPItello-291 clinical trial show that the combination doubled the median progression-free survival compared with the placebo in patients with HR-positive, HER2-negative advanced breast cancer.

BYLieve clinical trial data indicate long-term and very-long-term data disease control was observed in 25.6% and 16.5% of patients, respectively, with PFS at 24.8 and 29.4 months in patients with HR-positive, HER2-negative advanced breast cancer.

The presence of CD8+ cells may help predict outcomes for patients administered immune checkpoint inhibitors.
Efficacy and safety profile in combination with alpelisib is comparable to that reported in the BYLieve and SOLAR-1 clinical trials, according to a poster presentation at the San Antonio Breast Cancer Symposium.

Pharmacy Times will be covering the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, which gathers pharmacy professionals working in malignant and classical hematology in New Orleans, Louisiana.

Dawn L. Hershman, MD, MS, Columbia University Medical Center, New York, NY, presents her findings at the 2022 San Antonio Breast Cancer Symposium.
Clinical trial results found that second-line treatment with trastuzumab deruxtecan led to significantly longer overall survival compared with trastuzumab emtansine.
DESTINY-Breast02 clinical trial results show trastuzumab deruxtecan demonstrated a clinically meaningful and statistically significant improvement in progression-free and overall survival.

Study finds textured breast implants do not increase the risk of anaplastic large-cell lymphoma, which has grown in incidence since 2008.
Tumor microenvironment differences in Black women may explain disparities in outcomes for those with residual tumors after chemotherapy treatment for ER–positive/HER2-negative breast cancer.

Breast cancer expert joined a panel to discuss aromatase inhibitors at the San Antonio Breast Cancer Symposium (SABCS) on December 6, 2022.

Future research should investigate which therapies with new mechanisms of action add incremental benefit to existing treatment regimens for non-small cell lung cancer.
When used with chemotherapy, pembrolizumab provides a synergistic response to increase survival rates.
Phase 3 monarchE trial assesses distant relapse free-survival, invasive disease-free survival, and overall survival for the treatment of HR-positive/HER2-negative-, node-positive, high-risk early breast cancer.
RxPonder clinical trial results indicate that racial disparities continue to be a major health care challenge.
PHP shows promise extending survival in patients with inoperable intrahepatic cholangiocarinomas or extrahepatic cholangiocarinoma with liver metastases.

Results of RIGHT Choice phase 2 clinical trial show Kisqali doubled PFS and showed fewer adverse events.
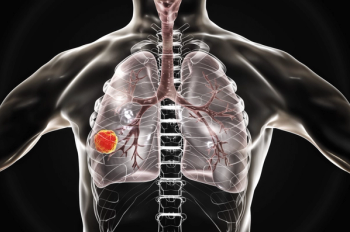
Biomarker testing and management of immune-related adverse events are key roles for pharmacists.

A recent study did not find a significant association between a healthy plant-based diet and the risk for colorectal cancer among 93,475 US women.

Three key opportunities for oncology stewardship are dose rounding, implementation of biosimilars, and management of sites of care.
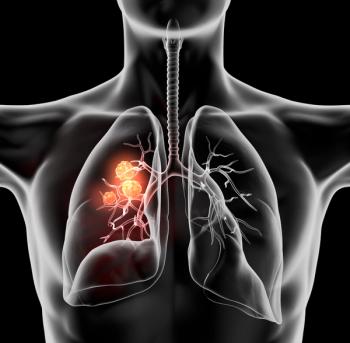
Scott Soefje, director of Pharmacy Cancer Care and assistant professor of Pharmacy at Mayo Clinic, discusses the role of PD-1 inhibitors in treating non–small cell lung cancer.
The allogeneic CAR-T cell therapy is in development for relapsed or refractory large B cell lymphoma and relapsed or refractory B cell non-Hodgkin lymphoma.
Nearly 8 out of 10 individuals within the recommended age range for colorectal cancer screening are considered to have an average risk for the disease.