
Heidi Finnes, PharmD, BCOP, FHOPA, discusses the evolving landscape of treatment of cutaneous malignancies.

Heidi Finnes, PharmD, BCOP, FHOPA, discusses the evolving landscape of treatment of cutaneous malignancies.

Jolynn Sessions, PharmD, BCOP, FHOPA, shares her experience as a clinical pharmacist and the value of the Oncology Pharmacists Connect meeting.

Staying current across every disease state, drug, and toxicity is burning people out.
At the 2025 Oncology Pharmacists Connect (OPC) meeting, Courtney Cavalieri, PharmD, BCOP, presented clinical trial findings in gastrointestinal (GI) cancers—including colon, gastric, pancreatic, and metastatic colorectal cancer—that highlight the expanding role of immunotherapy and targeted treatments.
At the 2025 Oncology Pharmacists Connect (OPC) meeting, Mya Tran, PharmD, BCOP, highlights the clinical promise, operational challenges, and evolving role of pharmacists in implementing precision medicine and genomics-driven cancer care.
Janet Espirito, PharmD, discusses real-world outcomes from a large community oncology study showing that patients with BRAF-mutated melanoma—including those with brain metastases—experienced similar benefit from frontline immune checkpoint inhibitor (ICI) therapy.
With robust pipelines, cell therapy remains the major driver for drug development.
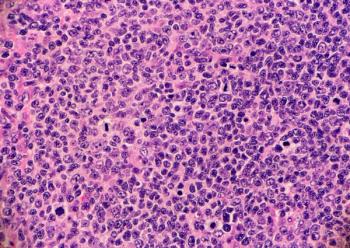
Following robust progression-free survival indications in the phase 3 inMIND trial, the FDA granted approval to tafasitamab-cxix in combination with standard-of-care lenalidomide and rituximab for patients with relapsed or refractory follicular lymphoma.
The emergence of biomarkers plays a critical role in the growing success of treatment.

In the first half of 2025, the FDA approved 7 novel drugs for oncologic conditions, including for non-small cell lung cancer; ovarian cancer; and metastatic, hormone receptor-positive, human epidermal growth factor 2-negative breast cancer.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.

This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.
This single-center, descriptive, retrospective chart review identified barriers to outpatient chemotherapy use, revealing avoidable inpatient stays and highlighting targets for stewardship interventions.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.
Bispecific therapies require clearer terminology to improve safety and communication.


Zahra Mahmoudjafari, PharmD, MBA, BCOP, FHOPA, co-chair of the 2025 Oncology Pharmacists Connect (OPC) meeting, discusses what makes the event unique, highlights key sessions, and shares her excitement for the educational and networking opportunities.

Manish Agrawal, MD, discusses emerging research on psychedelic-assisted therapy for patients with cancer, including innovative dyadic treatment models, challenges in capturing meaningful outcomes, and growing interest in integrating psychedelics into oncology and palliative care.

Ribociclib plus endocrine therapy shows improved survival in HR+, HER2- breast cancer, but its cost-effectiveness raises concerns in the US market.

Vitamin D could offer an alternative option over hard-to-access and burdensome cancer drugs to heighten the response of chemotherapy.

Ribociclib in combination with a nonsteroidal aromatase inhibitor (NSAI) improved invasive disease-free survival (iDFS) while allowing for better quality of life in hormone receptor-positive (HR+)/human epidermal growth receptor 2-negative (HER2−) early breast cancer.
The investigational blood test achieved 99% sensitivity and specificity.

Pharmacists are redefining health care by bridging clinical and economic evidence, making a real difference in patient outcomes through health economics and outcomes research (HEOR).
Pembrolizumab gains FDA approval as a groundbreaking neoadjuvant treatment for resectable locally advanced head and neck squamous cell carcinoma, enhancing patient outcomes.

Mitomycin intravesical solution is the first FDA-approved treatment for low-grade intermediate-risk non–muscle-invasive bladder cancer.

Biologics and biosimilars have a major role in modern medicine because of their clinical benefits and economic impact on patient care.