The designation is supported by pooled data from 4 clinical trials.

The designation is supported by pooled data from 4 clinical trials.
Nadofaragene firadenovec led to most patients with Bacillus Calmette-Guérin-unresponsive non-muscle invasive bladder cancer (NMIBC) experiencing a complete response (CR) at 3 months.
The FDA’s accelerated approval of lifileucel (Amtagvi; Iovance Biotherapeutics) marks a major milestone in immunotherapy for metastatic melanoma, building on decades of research in tumor-infiltrating lymphocyte therapy.
Immunotherapy pioneer James P. Allison, PhD, revolutionized cancer treatment by discovering immune checkpoint blockade, leading to significant advances in survival rates and ongoing efforts to optimize immuno-oncology strategies.
Advancements in neoantigen vaccines and immunotherapy are transforming cancer treatment by leveraging genomic sequencing to overcome tumor heterogeneity and therapeutic resistance.

Tanshinlactone is an active ingredient that is commonly used in Chinese herbal medicine.
BRAF V600E inhibitors show improved outcomes in relapsed/refractory hairy cell leukemia.
Melody Smith, MD, MS, discusses how microbiome modulation may influence CAR T-cell therapy toxicity and outcomes, highlighting potential future interventions and clinical integration challenges.
The AACR Immunotherapy Conference 2025 will provide pharmacists with the opportunity to engage with cutting-edge cancer immunotherapy research, explore advancements in treatments, such as immune checkpoint inhibitors and CAR T-cell therapy, and connect with key leaders in the field.
The approach targets cancer glucose and fatty acid metabolism, which is essential for cancer cell growth.
Melody Smith, MD, MS, discusses how the intestinal microbiome influences CAR T-cell therapy outcomes, highlighting the negative impact of certain antibiotics and the potential role of dietary interventions in improving treatment efficacy.

Axatilimab is a colony-stimulating factor-1 receptor–blocking monoclonal antibody indicated after 2 lines of systemic therapy.
67Cu-SAR-bisPSMA is indicated for adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC).

Using central obesity measures provides more accurate estimates of the proportion of CRC cases attributable to excess weight compared to using BMI alone.
Relapsed treatment has evolved with CAR T, bispecific antibodies, and immunotherapies.
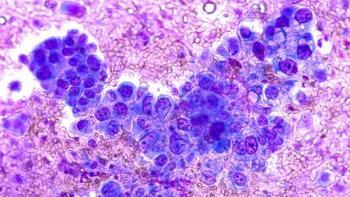
Priority review status will allow for expedited development of zongertinib, putting it on the path toward approval as the first in a new class of drugs for mutated NSCLC.
The 75 minutes of infusion time typically required is costly and difficult for patients.
T-DXd has a boxed warning for interstitial lung disease and pneumonitis.
Using new treatment advances, traditional medications, genetic profiles, immunotherapies, and individualized plans enables providers to improve outcomes for patients with TPBC.
The test offers an accurate, fast, low-cost, and noninvasive tool for early diagnosis of pancreatic ductal adenocarcinoma.

Treatment selection includes patient factors, cost, adverse effects, and drug interactions.
Second-line and later treatment options for small cell lung cancer are limited.
Nivolumab demonstrated a sustained disease-free survival benefit over placebo in high-risk muscle-invasive urothelial carcinoma (MIUC).
The data were presented at the 2025 ASCO Genitourinary Cancers Symposium.
The approval in multiple indications is designed to improve access to effective, proven treatments for skeletal fractures, which can greatly reduce patient quality of life.
DLL3 is an emerging therapeutic target in small cell lung cancer and other neuroendocrine carcinomas, with 1 FDA-approved therapy and several others under investigation.
C. Brooke Adams, PharmD, BCOP, discusses the evolving diagnostic criteria and management strategies for cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) in CAR T-cell therapy, highlighting advances in toxicity prevention and treatment selection.
Data from the phase 2 CABRAMET trial suggests the potential of cabozantinib for treatment of brain metastases from renal cell carcinoma (RCC).
Enfortumab vedotin, trastuzumab deruxtecan, and disitamab vedotin were highlighted at the 2025 ASCO Genitourinary Cancers Symposium.
Panelists at the 2025 ASCO Genitourinary Cancers Symposium discussed challenges and new approaches to treatment.