Studies suggest that prolonged activation of MUC1-C encourages disease progression.

Studies suggest that prolonged activation of MUC1-C encourages disease progression.

The combination demonstrated improved efficacy compared with ruxolitinib as a monotherapy.
Glofitamab combined with GemOx shows promising survival benefits for patients with relapsed DLBCL, offering a new treatment option for those ineligible for transplants.

Don Moore, PharmD, BCPS, BCOP, DPLA, FCCP, FASHP, received a leadership award at the 2025 HOPA Annual Meeting.

Pharmacists compare real-world practice data with clinical trial results and discuss supportive care options for patients.
A study reveals sasanlimab enhances event-free survival in high-risk bladder cancer patients, offering new hope against disease recurrence.
Pharmacists should continue to emphasize the vaccine’s effectiveness in preventing cancer and be prepared to address patient safety concerns.
Naxitamab-gqgk has been added as a Category 2A recommendation in the updated National Comprehensive Cancer Network (NCCN) guidelines for high-risk neuroblastoma, reflecting growing consensus on its role in treating relapsed or refractory disease.
Cynthia Ryan, PharmD, BCPS, discusses practical, evidence-based strategies for pharmacists to identify, prevent, and manage dermatologic toxicities associated with cancer therapies, emphasizing interdisciplinary collaboration, patient education, and proactive supportive care to improve adherence and outcomes.
New data reveals that combining pertuzumab with trastuzumab significantly improves survival rates in HER2+ breast cancer patients, reducing death risk by 17%.
The regimen, which consists of durvalumab and Bacillus Calmette-Guerin, offers new hope to patients with high-risk non-muscle-invasive bladder cancer.

The FDA approves retifanlimab for advanced anal cancer, offering new hope with improved survival rates and treatment options for patients.
Combining olaparib with neoadjuvant chemotherapy enhances survival rates for early-stage gBRCAm triple-negative breast cancer, offering new hope for patients.
In patients with synchronous metastatic colorectal cancer treated without curative intent, high low-density lipoprotein (LDL) cholesterol was associated with poor survival.
The data are from the phase 2 iMMagine-1 study.
Emrelis is a groundbreaking treatment for advanced non-small cell lung cancer, targeting high c-Met protein overexpression.
The FDA approved belzutifan, the first oral treatment for pheochromocytoma and paraganglioma, offering a new treatment pathway for patients with these rare tumors.

Effectively communicating research findings to patients, health care professionals, and policy payers is necessary for engagement.

This new generic will impact both oncology and community pharmacists.

Explore the latest insights on emerging antibody drug conjugates for HER2 breast cancer and real-world management strategies.

During a panel discussion, 3 experts highlighted ways health care professionals and researchers can help provide patient-centered care.

The new at-home test aims to increase comfort and screening timeliness through a more accessible method.
Abemaciclib and ribociclib have various risks and benefits in treating early breast cancer, highlighting the importance of patient-provider communication.

With the FDA approval, avutometinib/defactinib is now the first and only FDA-approved treatment for patients with recurrent low-grade serous ovarian cancer (LGSOC).

Pharmacists can educate patients on nonpharmacologic strategies that help improve survival outcomes.
The agent demonstrated promising tolerability and manageable safety.
Trastuzumab deruxtecan was safe and efficacious prior to treatment with paclitaxel, trastuzumab, and pertuzumab.

The time frame concept is an underappreciated through line in most planning efforts.

The agent targets B7-H3, a commonly overexpressed protein in diffuse intrinsic pontine glioma (DIPG).
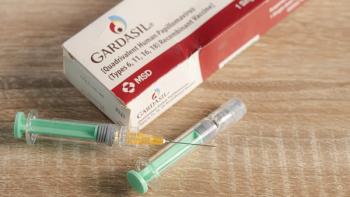
One dose of the vaccine compared with 2 may help increase global HPV vaccine uptake.