
The FDA has approved the PD-L1 IHC 22C3 pharmDx assay, developed by Agilent Technologies Inc, for expanded use in patients with non–small cell lung cancer.

The FDA has approved the PD-L1 IHC 22C3 pharmDx assay, developed by Agilent Technologies Inc, for expanded use in patients with non–small cell lung cancer.
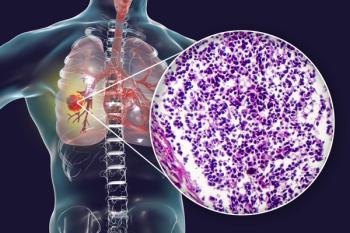
The time to treatment deterioration in pain in chest, dyspnea, and cough was found to be comparable between patients who received lorlatinib and patients who received crizotinib.
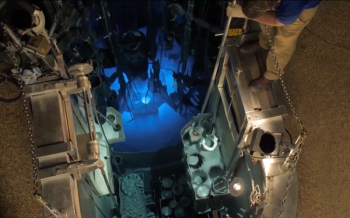
Pharmacy Times® interviewed J. David Robertson, PhD, director of University of Missouri Research Reactor, on a new collaboration with Advanced Accelerator Applications International to develop a targeted therapy for certain types of cancerous tumors.
Two expert speakers at the 2020 ASHP Midyear Clinical Meeting and Exhibition presented Immune Checkpoint Inhibitors: Resolving Dosing Confusion to Better Care for Patients With Cancer, a virtual symposium.
Statins have become a standard part of treatment for certain patients with cancer to prevent cardiotoxicity and promote antitumor activity.
The FDA has granted a fast track designation to the DNA-mediated interleukin-12 immunotherapy GEN-1 for use in the treatment of patients with advanced ovarian cancer.

This is the third approval for Libtayo, following approvals as the first immunotherapy for patients with advanced basal cell carcinoma and as the first systemic treatment for advanced cutaneous squamous cell carcinoma.
A deep learning model was found to support the identification of imaging biomarkers on screening mammograms, according to researchers at Massachusetts General Hospital.

This week on Pharmacy Times, there are a number of important topics that will be covered and posted throughout the week.

As a direct result of the global pandemic, the landscape for oncology health care professionals has changed.

Current cancer research focuses heavily on adjunct immunotherapy in combination with traditional chemotherapy.
A 5% weight loss over 2 years in patients with HER2-positive early breast cancer was associated with worse outcomes, according to new research that investigated the BMI data of these patients.
Presentation provides an overview of recent FDA approvals for therapies utilized in patients with various cancer types.

Two supplemental BLAs have been submitted to the FDA for enfortumab vedotin-ejfv to convert its accelerated approval into a regular one and to expand the current label to include patients with locally advanced or metastatic urothelial cancer.
The classes of approved cell and gene therapies are expected to expand rapidly in the next few years based on projections in the field.

The FDA granted a priority review designation to the biologics license application for Vicineum for use in patients with high-risk Bacillus Calmette-Guérin–unresponsive non–muscle invasive bladder cancer.
A recent comprehensive, prospective study of smoking habits among patients with non-small cell lung cancer demonstrated a high rate of smoking reduction and cessation following entry into the phase 3 early-stage trial.

Medication reconciliation is a critical component of the oncology pharmacistʼs duties that has been performed safely over the phone with the patient, caregiver, and pharmacy with the assistance of electronic health records.
The FDA has granted priority review to sotorasib for the treatment of patients with KRAS G12C–mutated locally advanced or metastatic non–small cell lung cancer, after at least 1 previous systemic therapy.
Data from the FIGHT trial suggests that approximately 30% of patients with non-human epidermal growth factor 2-positive GEJ cancers overexpress fibroblast growth factor receptor 2.

Educating patients about cervical cancer and the HPV vaccine can help reduce the number of cases.

Our cover article focuses on notable oncology trends for 2021.

Fractional CO2 laser therapy may offer a nonhormonal treatment to improve sexual function in survivors of breast cancer with genitourinary syndrome of menopause.

The FDA has approved trilaciclib (Cosela) to reduce the frequency of chemotherapy-induced bone marrow suppression in adults receiving certain types of chemotherapy for extensive-stage small cell lung cancer.

Investigators found that poor diets are particularly common among adult cancer survivors who have significant sociodemographic disparities, including those who have less formal education and those who are overweight.

Data show that 1 in 5 men and women worldwide develop cancer during their lifetime, and 1 in 8 men and 1 in 11 women die from the disease, according to the study.
The efficacy results demonstrated that there were 4 patients with confirmed partial response, and an additional 3 patients had stable disease lasting 16 weeks or longer.
A biologics license application has been submitted to the FDA for the accelerated approval of tisotumab vedotin for use in patients with recurrent or metastatic cervical cancer that has progressed on or following chemotherapy.

Pharmacy Times interviewed Jason Valent, MD, of the Cleveland Clinic the next steps for his CAEL-101 findings, a treatment for patients living with amyloid light chain amyloidosis.
Libtayo is the first treatment to show a clinical benefit in patients with advanced basal cell carcinoma after hedgehog pathway inhibitor therapy in a pivotal trial.