
The lingering effects of the COVID-19 pandemic, including supply concerns and staffing shortages, have made technological solutions become more crucial.

The lingering effects of the COVID-19 pandemic, including supply concerns and staffing shortages, have made technological solutions become more crucial.
Ronald T. Piervincenzi, CEO of US Pharmacopeia (USP), discusses recent survey data demonstrating 90% of physicians had experienced an erosion of trust in the US supply chain’s ability to deliver safe, quality medicines.
Edoxaban (Savaysa) can reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.

Tiago Reis Marques, MD, PhD, CEO of Pasithea Therapeutics, addresses how pharmacists can respond to questions patients may ask them about ketamine and other psychedelic treatments.

Tiago Reis Marques, MD, PhD, CEO of Pasithea Therapeutics, explains how ketamine infusions work when administered in patients’ homes and what safety precautions would be taken by providers in this setting.

A variety of drugs from several medication classes are under investigation.

New composite pharmacy adherence measure is a major step in developing standard pharmacy performance measures focused on improving patient care.

Study shows that treatment with rituximab (Rituxan) for B-cell non-Hodgkin lymphoma prior to COVID-19 vaccination significantly lowered the number of patients who developed blocking antibodies for the virus.

Lack of immediate results, cultural barriers, and high costs lead many to gamble with their cardiovascular health, study results show.
The calculation instrument displayed good discrimination, with a C-index of 0.72, as well as good calibration, investigators say.

Data suggest that omicron-specific CD8-positive T cell responses were more than 80% cross-reactive with the CD8-positive T cell response to the original strain of the virus.

Larotrectinib was the first drug to receive accelerated FDA approval for patients of all ages based on a common molecular marker, independent of tissue origin for targeted therapy.

Applying these findings in practice could improve clinicians’ ability to offer truly personalized cancer therapy by enabling consideration of toxicity along with other data predicting patients’ responses.
Fecal microbiota transplantation produces cure rates of 82%-88% in patients with recurrent clostridium difficile infections.
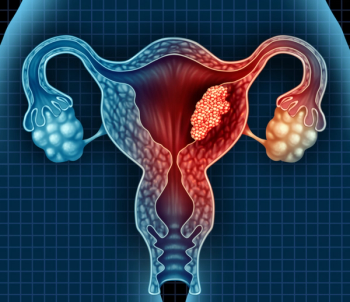
Selinexor demonstrated a 37% increase in probability that patients treated with the drug will be in remission at 12 months.

The use of a unique trimer to produce tier-2 neutralizing antibodies may help fight HIV.

Sutimlimab-jome is the only approved treatment for individuals with CAD and works by inhibiting the destruction of red blood cells.

The agency has also approved the baclofen oral suspension for Azurity Pharmaceuticals to treat individuals with other spinal cord diseases or injuries.
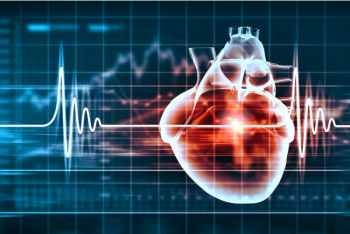
Following the first 3 days after a COVID-19 diagnosis, the risk of stroke quickly decreased.
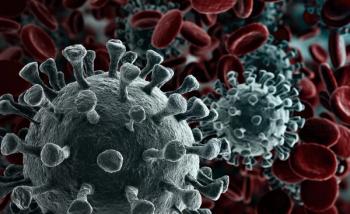
Monoclonal antibodies are highly effective at treating the virus, but they have often gone to the healthiest patients, instead of those over aged 65 years.
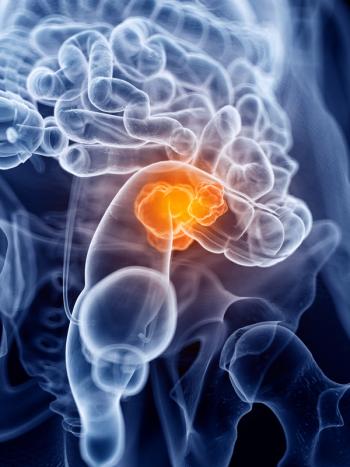
Culturally tailored patient navigation can improve underserved individuals’ health literacy, trust in the health care system, and use of cancer screening tools, study results show.

The results of a pilot program investigating the efficacy of remote patient monitoring for patients with cancer showed that it helped to keep high-risk individuals out of the hospital.

Communication, flexibility, and virtual solutions will remain key as the COVID-19 pandemic continues.
Trametinib emerged as a novel therapeutic approach for low-grade serous carcinoma due to a high prevalence of activating mutations in the mitogen-activated protein kinase signaling pathway.

Millions of people are still getting COVID-19, so the need for additional tools to fight the virus remains strong.

But 3 main challenges stand in the way of their adoption: patent litigation, patient and physician concerns, and rebates and reimbursements.
Investigators also found that intravenous immunoglobulins tend to be associated with increased frequency, though not significant, of serious adverse events.
Pfizer and BioNTech are seeking an Emergency Use Authorization for their COVID-19 vaccine to include children 6 months through 4 years of age.
Although the researchers were able to find an association between a heart attack and faster cognitive decline, the findings did not establish that a heart attack directly causes cognitive decline.

Greenstick fractures occur most commonly in children because their bones are softer and more flexible than the bones of adults.