
A number of prescription medications are indicated for the treatment of this aggressive disease.

A number of prescription medications are indicated for the treatment of this aggressive disease.

Polatuzumab vedotin regimen is the first in 2 decades to show such significant improvements.
The partial immune response observed in these patients can create an environment for immune selection of evolutionary variants, according to the authors of the study.
Ten quiz questions to assess your knowledge on common multiple sclerosis treatments.

Multimorbidity, health-related quality of life, and stigma and discrimination continue to be major issues for people living with HIV, including those who have achieved viral suppression, according to a consensus statement from a multidisciplinary panel of HIV experts published in Nature Communications.

The investigators found that the combination of cemiplimab-rwlc and chemotherapy resulted in a substantial improvement in OS compared to chemotherapy alone for patients with metastatic or locally advanced disease and tumors with either squamous or non-squamous histology and across all programmed death-ligand 1 expression levels.
Asparaginase erwinia chrysanthemi (recombinant)-rywn) (Rylaze) is approved for use as a component of a chemotherapy regimen for the treatment of acute lymphoblastic leukemia and lymphoblastic lymphoma

Research presented at the Alzheimer’s Association International Conference (AAIC) 2021 found associations between COVID-19 and persistent cognitive deficits, including the pathology and symptoms of Alzheimer disease.
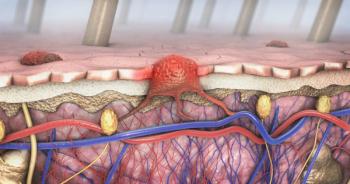
BCC is the most common form of skin cancer and is typically treated with surgical excision. However, excision can be a costly and burdensome treatment, especially for patients with multiple BCC lesions, according to the study.
Truvada is a medicine people at risk for HIV should take to prevent infection with the virus from various ways, such as sex or injection drug use.
C difficile colonization rates among children were greatest at 6 to 12 months of age but decreased among children older than 5 years of age.

Avalglucosidase alfa-ngpt is indicated for patients aged 1 year and older with late-onset Pompe disease.

A stepwise approach to treatment for multisystem inflammatory syndrome, beginning with initiation of immunomodulatory drugs, was successful in all patients included in the study.
Patients with COVID-19 who were vaccinated against the flu were found to be significantly less likely to visit the emergency department and be admitted to the intensive care unit.

Findings reflect fewer visits to health facilities, social distancing, and other safety measures implemented during the pandemic.
Plinabulin is a selective immunomodulating microtubule-binding agent (SIMBA), which is an antigen presenting cell (APC) inducer.

A generic TDF/FTC costs as low as $30 per month, compared with about $1800 per month for branded TAF/FTC
Study examines whether dapagliflozin is cost-effective when added to standard of care for patients who have heart failure with reduced ejection fraction.

According to the investigators, the findings demonstrate that a 5-day regimen of stereotactic body radiotherapy, a form of external beam radiation therapy that uses a higher dose of radiation, had a 4-year cure rate of 82%.
Although there was a numerical difference between the mean number of new or newly enlarging T2 hyperintense lesions at week 72 of 0.05 (Q4W) and 0.20 (Q6W), this difference was not clinically meaningful in the context of the full data, according to the investigators.

ACE inhibitors are currently prescribed more commonly than ARBs as a first-time blood pressure control medicine despite this difference in AEs, the study authors noted.

According to a press release from Roche, the FDA is reviewing the company’s Biologics License Application under the Real-Time Oncology Review pilot program, which aims to explore a more efficient review process to ensure safe and effective treatments are available to patients as early as possible.

This month, I thought it would be beneficial to share a handful of examples from among the many Next Generation Pharmacist-nominated candidates.

MDM2 inhibitors and BET inhibitors, which show limited efficacy against acute myeloid leukemia (AML) as monotherapies, are potent against AML when used in combination, according to a study published in Nature Communications.
The OVATION 2 study combines GEN-1 with standard-of-care neoadjuvant chemotherapy (NACT) in patients newly diagnosed with stage 3 and 4 ovarian cancer.

Anna Legreid Dopp, PharmD, CPHQ, senior director of clinical guidelines and quality improvement at ASHP, said their organization was proud to sign onto a recent statement urging health care organizations to mandate COVID-19 vaccines for their employees.
The investigators also predicted that the increase in indications of novel diabetes treatments and their increased use is likely to result in greater expense.

Pharmacy Times interviewed Craig Freyer, PharmD, BCOP, and Andrew Lin, PharmD, BCOP, to discuss how to manage the logistical challenges that arise during the administration of CAR T therapy in inpatient and outpatient settings for patients with multiple myeloma.

The analysis used AstraZeneca’s global safety database, which contains all spontaneously reported adverse events from real-world use of its medicines and vaccines worldwide.

In addition to getting outside and taking time to enjoy hobbies outside of work, Carroll urged pharmacists to enjoy time with family and friends whenever possible.