
Incorporating mental distress screenings during cancer care have been historically difficult, despite the fact that these patients tend to be vulnerable to mental health challenges.

Incorporating mental distress screenings during cancer care have been historically difficult, despite the fact that these patients tend to be vulnerable to mental health challenges.
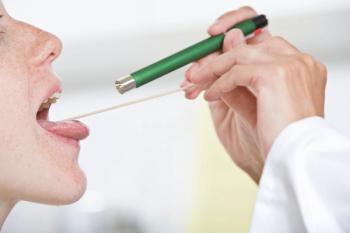
Despite best practice recommendations, prescriptions for acute pharyngitis are frequently not accompanied by testing, Epic Research data show.

Point-of-care testing can vary in complexity, but is always performed at or near a patient and at the site where care or treatment is provided.
Study results establish LAG-3 inhibition as therapeutically relevant third immune checkpoint pathway
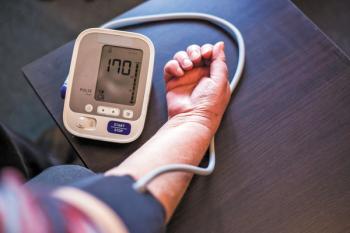
Additionally, the study showed a 15-fold increase in maternal mortality rates associated with chronic hypertension over the 40-year study period.

Alyson McGregor, MD, of Brown University explains what pharmacists should understand about the impact of sex and gender on medicine within their practice.
Proof-of-contest is inexpensive and quantifies specific antigens from a drop of blood within minutes.

Patients with EGFR exon 20 insertion mutation NSCLC account for approximately 1%-2% of NSCLC cases.

GeneSight Mental Health Monitor finds that many struggle to know the difference between teenage behavior and a mental health challenge.

Results of a separate analysis demonstrate that Johnson & Johnson’s booster generated a 41-fold increase in neutralizing antibodies and a 5-fold increase in T-cells against omicron.

Alyson McGregor, MD, of Brown University explains what pharmacists can do to advance issues arising around the impact of sex and gender on medicine.
The submission is supported by data from MajesTEC-1, a multicenter clinical trial evaluating the safety and efficacy of teclistamab in adults with RRMM.
In laboratory molecular tests, scientists mix samples with assay reagents in a highly controlled process, but because the reaction conditions are tightly controlled, the tests are both sensitive and selective, according to the researchers.

These lumps can relate to the overproduction of hormones, including the stress steroid hormone cortisol, which can lead to T2D and high blood pressure.

Rebecca Sleeper, PharmD, FCCP, FASCP, BCPS, of the Texas Tech University Health Sciences Center Jerry H. Hodge School of Pharmacy, discusses how a lack of sex and gender specific patient care education can impact a pharmacist’s work with their patients.
In the first quarter of 2022, Genprex expects to initiate its Acclaim-2 clinical trial to evaluate the treatment for individuals with histologically confirmed unresectable stage III or IV non–small cell lung cancer.
Tixagevimab co-packaged with cilgavimab is one of the only 2 antibody therapies issued emergency use authorization by the FDA that showed neutralizing activity against omicron and all other variants of concern.
The results of an analysis from investigators from Baylor College of Medicine suggest that supplementation with GlyNAC might be beneficial to those who have the virus.

The metabolic protein SIRT3 reduces the pools of amino acids that cells use to make proteins and otherwise fuel their growth.

Alyson McGregor, MD, of Brown University discusses the focus of her research into sex and gender in emergency medicine.
Telisotuzumab vedotin (Teliso-V, AbbVie) is an investigational antibody-drug conjugate that targets c-Met, a receptor tyrosine kinase that is overexpressed in tumors.
The approval marks the first small interfering RNA therapy for low-density lipoprotein cholesterol reduction.
The study analyzed blood samples taken from individuals infected with COVID-19, those who had been vaccinated with a 2-dose schedule and a third booster dose, and those who had reported previous infection from other COVID-19 variants of concern.

This is an update to the 2011 AAN guideline on the treatment of painful diabetic neuropathy, according to a press release.

When using a therapy to clear the senescent cells, disease progression and cell death were stopped, according to the study.

The existing EUA was also amended to reduce the time for booster dose administration from a minimum of 6 months to at least 5 months following completion of the primary COVID-19 vaccine series.
The Breakthrough Therapy Designation was granted based on data from the dose escalation portion of 2 expansion cohorts of a 3-cohort phase 1 study.

Results from a study conducted by the Institute for Health Metrics and Evaluation show that new cases jumped 26.3% over the same period.
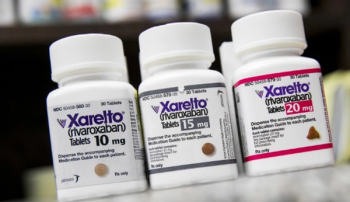
Rivaroxaban is indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, for the treatment of deep vein thrombosis, and pulmonary embolism.
Small GISTs may cause no symptoms and may grow so slowly that they have no serious effects.