
Triumeq is a tablet containing 50 mg of dolutegravir, 600 mg of abacavir, and 300 mg of lamivudine.

Triumeq is a tablet containing 50 mg of dolutegravir, 600 mg of abacavir, and 300 mg of lamivudine.

Heidi D. Finnes, PharmD, BCOP, FHOPA, president-elect of Hematology/Oncology Pharmacy Association (HOPA), discusses ways to address burnout in the hematology/oncology space.
FDA to evaluate New Drug Application for futibatinib in the treatment of patients with previously treated locally advanced or metastatic cholangiocarcinoma.
The Roche subsidiary reports that it will continue to evaluate tiragolumab plus Tecentriq and chemotherapy in non-small cell lung cancer and other cancer types through more phase 3 trials.

Heidi D. Finnes, PharmD, BCOP, FHOPA, president-elect of Hematology/Oncology Pharmacy Association (HOPA), provides details of the upcoming HOPA 2022 conference.

ImmunoGen, Inc submits Biologics License Application under the accelerated approval pathway for mirvetuximab soravtansine monotherapy for patients with FRα-high platinum-resistant ovarian cancer previously treated with 1 to 3 prior systemic treatments.

They are also at greater risk of death than men when undergoing cardiac procedures, a new analysis shows.

Analysis from the American Society of Health-System Pharmacists indicates turnover rates of at least 21% in 2021.

FDA approves expanded indication for the treatment of HIV-1 in virologically suppressed adolescents 12 years of age or older, who weigh at least 35 kg, and who are on a stable antiretroviral regimen, with no history of treatment failure or known or suspected resistance to either cabotegravir or rilpivirine.

Veyonda, a novel proprietary formulation of idronoxil, is a first-in-class, dual-acting oncotoxic and immuno-oncology molecule.

Elizabeth Spurlock, MA, PHR, director of HR Business Partner at Texas Oncology, discusses how burnout among physicians, nurses, and hospital administrators has impacted the role of the pharmacist in patient care.

Heidi D. Finnes, PharmD, BCOP, FHOPA, president-elect of Hematology/Oncology Pharmacy Association (HOPA), provides details of the upcoming HOPA 2022 conference.

An 11-fold increase in geometric mean neutralizing antibody titers was reported at 2 weeks after the second booster compared to 5 months after the first booster dose.

Patient treatment improves and medication prices decline as a result, a new analysis indicates.
RBX2660, a standardized, stabilized, novel microbiota-based live therapeutic, shows promise treating patients with recurrent Clostridium difficile infection.
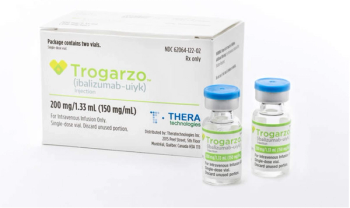
Ibalizumab-uiyk (Trogarzo) is a CD4-directed post-attachment HIV-1 inhibitor.
Abemaciclib (Verzenio; Lilly) is the first addition to adjuvant endocrine therapy approved by the FDA in two decades.

Access to buprenorphine among Medicaid beneficiaries was not affected during the pandemic.
Electronic, open loop, clinical decision support system helped to produce a 38% lower overall mortality rate among patients with pneumonia.
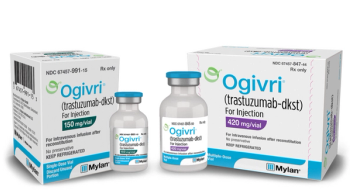
Trastuzumab (Herceptin, Herzuma, Kanjinti, Ogivri, Ontruzant) is a HER2/neu receptor antagonist indicated for early and advanced breast cancer, advanced stomach cancer, and gastroesophageal junction cancer.
Acalabrutinib (Calquence; AstraZeneca) provides efficacy while maintaining favorable tolerability for patients with chronic lymphocytic leukemia.

FDA seeks to reduce diversion of pharmaceutical products via sales by unlicensed distributers and tighten up the supply chain by vetting trading partners.

Heat waves, which are on the rise, appear to pose particular cardiovascular risk for Black populations and men, new study results show.

FDA approves label update for Cabenuva that makes the therapy’s oral lead-in with cabotegravir and rilpivirine tablets optional.

Larry Buie, PharmD, BCOP, FASHP, outgoing president of the Hematology/Oncology Pharmacy Association (HOPA), shares some details of HOPA’s upcoming 2022 annual conference in Boston and provides an overview of his term as HOPA’s president.
Investigators also identified that a polymerase inhibitor with a unique modification largely resists its removal from the RNA by the exonuclease.

Osimertinib (Tagrisso; AstraZeneca) is FDA approved for 3 unique cancer indications.

Leveraging automation capabilities and operational capacity at scale, they bridge gaps and mitigate the impact of disruptions.

Pluvicto is indicated for the treatment of adults with prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer who were previously administered other anticancer therapies.
Bevacizumab (Avastin) is a vascular endothelial growth factor inhibitor indicated several cancer types.