Simplifying processes reduces errors in compounding, IV pump programming, and physician order entry.

Simplifying processes reduces errors in compounding, IV pump programming, and physician order entry.

Nakia Eldridge, PharmD, MBA, director of health care quality, safety, and information at US Pharmacopeia (USP), discusses points of note regarding USP’s work in setting and supporting public quality standards.

Does sufficient evidence exist to support an award against pharmacists’ employer, a national pharmacy chain?

When employees hint or openly share that they are feeling overextended, managers should acknowledge their concerns and work toward creative solutions.
Intravenous immunoglobulin shows promise as a treatment option for pregnant patients with SARS-CoV-2, influenza, and other respiratory viruses.

Goal of modernizing therapeutic equivalence rating determination act is to increase availability, competition, drive down prescription costs.
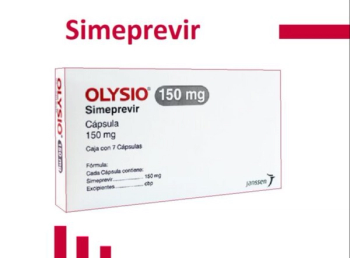
:Simeprevir (Olysio) is an NS3/4A protease inhibitor indicated for the treatment of chronic hepatitis C infection as a component of a combination antiviral treatment regimen.

Antibody levels against SARS-CoV-2 1 month after the booster dose were increased compared to before the booster dose in children 5 to 11 years of age.

The FDA previously placed a clinical hold on lenacapavir in borosilicate vials in all clinical studies because of emerging concerns about the compatibility of vials made of borosilicate glass.

Analysis shows the activity of a specific enzyme, Butyrylcholinesterase, was significantly lower in those who subsequently died of sudden infant death syndrome.
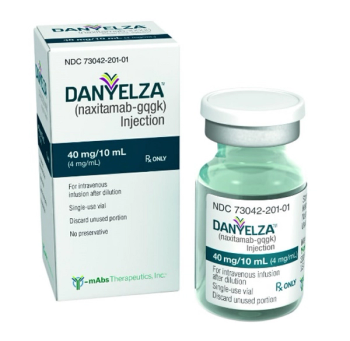
Naxitamab-gqgk (Danyelza) is for the treatment of pediatric and adult patients with relapsed or refractory high-risk neuroblastoma in the bone or bone marrow who have demonstrated a partial response, minor response, or stable disease to prior therapy.

Ribociclib plus fulvestrant achieved a median overall survival of 67.6 months in the first line setting for postmenopausal women with HR-positive/HER2-negative advanced breast cancer.
Infections at community hospitals fell by 63% after 1 year and 77% after 3 years, new data show.

Individuals who have recovered from the initial disease are in generally poorer health later on compared with the general population, the analysis shows.
Asundexian was granted FDA Fast Track Designation based on a phase 2 study evaluating its safety and efficacy combined with antiplatelet therapy in patients following an acute, non-cardioembolic ischemic stroke.

Health care policy decisions based on sound data and research are essential for addressing the challenges.
Many of patients with hepatitis that pharmacists encounter have viral hepatitis, which can be easily remembered as the ABCs of hepatitis.
Atezolizumab (Tecentriq) is indicated for use in urothelial carcinoma, non-small cell lung cancer, triple-negative breast cancer, small cell lung cancer, hepatocellular carcinoma, and melanoma.

The findings suggest that the broad use of race as a factor for providing certain health interventions and treatments should be reconsidered, investigators say.

Aklilu Tedla, vice president of strategy and business development at Cardinal Health, discusses the cell and gene therapies in the pipeline for approval by the FDA.

Organizations should focus on improving employee well-being and reducing workplace stressors.

Aklilu Tedla, vice president of strategy and business development at Cardinal Health, discusses the future of pharmacies as more cell and gene therapy products become available to patients.

Scientific abstracts also address findings on cardiovascular disease and population, the connection between COVID-19 and HF, and links between prognosis and marital status.

The advisory, published in Circulation, is a call to action for a cultural shift to identify and remove barriers.

Repotrectinib awarded breakthrough therapy designation for patients with ROS1-positive metastatic non–small cell lung cancer previously treated with a ROS1 tyrosine kinase inhibitor and who were not previously administered platinum-based chemotherapy.
The SKYSCRAPER-01 trial evaluating the drug plus Tecentriq demonstrates that it did not meet its co-primary endpoint of progression-free survival.

They should consider many factors and use clear guidance on predicting phenotypes from genotypes to intervene optimally based on specific drug-gene interactions.

Delafloxacin (Baxdela) is a fluoroquinolone antibacterial indicated for the treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia caused by designated susceptible bacteria.
Neil Lund, senior advisor, Avelere Health and Ryan Urgo, managing director, Health Policy, Avelere Health, discuss the impact of President Biden’s health care agenda on the pharmacy field.

Doug Long, vice president, Industry Relations, IQVIA, discusses notable trends reshaping specialty pharmaceutical channels and the impact of these developments on the role of the pharmacist in the health care team.