Lipophilic statins for dyslipidemia penetrate muscle more easily than hydrophilic statins and are associated with a higher incidence of adverse effects.

Lipophilic statins for dyslipidemia penetrate muscle more easily than hydrophilic statins and are associated with a higher incidence of adverse effects.
Blood pressure has to be monitored closely in patients diagnosed with stage 1 or 2 hypertension.
Bezlotoxumab is administered to prevent Clostridium difficile infection from recurring in patients who are receiving treatment for the infection and are at risk for recurrence.
Older drugs such as lovastatin (Mevacor, Altoprev), simvastatin (Zocor), and atorvastatin (Lipitor) have the most drug interactions.
Heart failure outcomes improve with optimal iron levels.
Ten quiz questions to assess your knowledge on common diabetes treatments.
Aldosterone blockers, such as spironolactone and eplerenone, can cause hyperkalemia.
Below a certain GFR, clinically significant reductions in plasma glucose cannot be achieved with sodium-glucose transport protein 2 inhibitors.
How should pharmacists manage a patient with diabetes on metformin with GFR below 30?
Zydelig (idelalisib) is a kinase inhibitor approved in 2014 for 3 oncology indications.

Ten quiz questions to assess your knowledge on common treatments and counseling points for heart failure.

Cabozantinib (Cabometyx, Exelixis) is a kinase inhibitor approved for indications such as advanced renal cell carcinoma and hepatocellular carcinoma.
Brexucabtagene autoleucel (Tecartus, Kite Pharma) is a cell-based gene therapy for the treatment of adult patients with mantle cell lymphoma who have not responded to or who have relapsed following other treatments.

Ten quiz questions to assess your knowledge on common hypertension treatments.

Margetuximab-cmkb (Margenza) was approved in December 2020 in combination with chemotherapy for the treatment of adult patients with metastatic HER2-positive breast cancer who have previously received 2 or more anti-HER2 regimens, at least one of which for metastatic disease.

Education and preparedness are essential for the treatment of this inherited disorder related to the musculosketal system.

Other options for Crohn disease and ulcerative colitis include biologics, corticosteroids, immunosuppressants, and vedolizumab.
Panzyga is indicated for the treatment of primary humoral immunodeficiency, chronic inflammatory demyelinating polyneuropathy, and chronic immune thrombocytopenia.

Sacituzumab govitecan is approved for the treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer who have received 2 more prior systemic therapies.

Lactitol (Pizensy) is an oral solution for treating chronic idiopathic constipation in adults.

Umbralisib was granted accelerated approval by the FDA for the treatment of select patients with relapsed/refractory marginal zone lymphoma and relapsed/refractory follicular lymphoma.

Trilaciclib (Cosela) is indicated to reduce the frequency of chemotherapy-induced bone marrow suppression in adults receiving certain types of chemotherapy for extensive-stage small cell lung cancer.
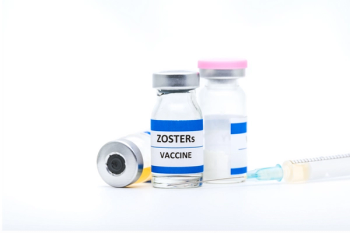
Antiviral medications, including Acyclovir, famciclovir, and valacyclovir, are effective if taken as soon as the diagnosis and can shorten the duration of the infection while improving symptoms.

Azacitidine (Onureg) was approved by the FDA in September 2020 as a maintenance therapy for acute myeloid leukemia.
The demand for drug treatments in Europe and North American has increased in recent years, as awareness of the bleeding disorder rises.

Xembify (immune globulin subcutaneous human–klhw) is a 20% immune globulin indicated for treatment of primary humoral immunodeficiency disease in patients 2 years of age and older.
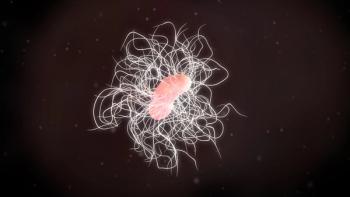
Pharmacists should educate themselves about the options available to help patients manage this bacterium that causes severe diarrhea and colitis.

Earlier this year, the FDA granted accelerated approval to tepotinib (Tepmetko, EMD Serono Inc) for the treatment of adults with metastatic non-small cell lung cancer who are harboring MET exon 14 skipping alterations.

The FDA recently approved the amyloid beta-directed antibody indicated for the treatment of the progressive disease.
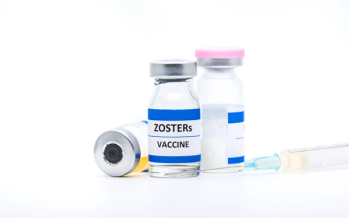
Shingrix is a non-live, recombinant subunit vaccine given intramuscularly in two doses for the prevention of herpes zoster in adults aged 50 years and older.