
Anorexic patients may not experience the reward-based motivation for food that drives many individuals to eat, which would explain how they are able to control their appetite.

Anorexic patients may not experience the reward-based motivation for food that drives many individuals to eat, which would explain how they are able to control their appetite.

An oral formulation of deferasirox is approved for the treatment of chronic iron overload due to blood transfusions.

Medtronic's CoreValve System is now approved for first-of-its-kind "valve-in-valve" replacement.

Ferumoxytol (Feraheme) has been responsible for a number of serious reactions and deaths since its 2009 approval.

The FDA today approved a new administration option for ticagrelor (Brilinta).

The FDA today approved anthrax immune globulin intravenous (Anthrasil) for the treatment of inhalational anthrax in combination with appropriate antibiotics.

Tighter hydrocodone regulations meant to reduce opioid abuse are hindering pain patients' access to the products.

For Jillian Beneventi, a pharmacy student at the Midwestern University Chicago College of Pharmacy, no element of her work as a future pharmacist is as important as the relationships she builds with her patients.
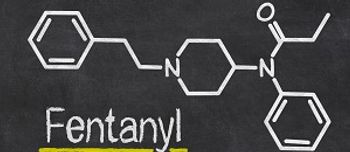
In response to a recent resurgence in fentanyl abuse, the DEA issued a nationwide alert about the dangers of the drug, describing it as a threat to health and public safety.
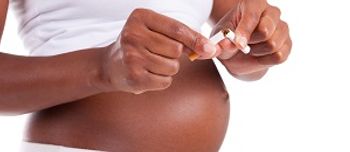
Women who smoke during pregnancy may be putting their children at risk for developing diabetes in adulthood.

Orexo has recently announced that the 8.6-mg/2.1-mg dosage strength of its partial opioid agonist, buprenorphine and naloxone (Zubsolv), is now available in the United States.

The National Institutes of Health today admitted an American healthcare worker who tested positive for Ebola virus while volunteering at an Ebola treatment unit in Sierra Leone.

Loved ones, friends, and health care providers of individuals whom they believe to be at risk of suicide will soon have access to new tools developed by Facebook and SAMHSA that will enable them to better help those in need.
Boehringer Ingelheim recently submitted a biologics license application to the FDA requesting accelerated approval of its investigational drug, idarucizumab, for the rapid reversal of anticoagulant effects of dabigatran (Pradaxa).
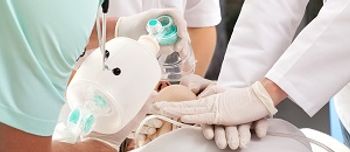
The FDA today approved a CPR device that the agency claims may improve a patient's chances of surviving cardiac arrest.
Hospira is voluntarily recalling a mislabeled lot of its injectable anti-seizure medication, magnesium sulfate in 5% dextrose, as well as 1 lot of its 0.9% sodium chloride injection, following a confirmed report of particulate in a single unit.
The FDA today authorized the use of the first device approved to treat dialysis-related amyloidosis.

Sagent Pharmaceuticals is voluntarily recalling 6 lots of its atracurium besylate injection following FDA observations of practices at the manufacturer's site that could potentially affect the products' sterility.

New app has wide array of services for pharmacists.
Opdivo was originally approved for patients with unresectable or metastatic melanoma who no longer respond to other drugs.
The FDA announced today that it has expanded the indication of Bristol-Myers Squibb's anticancer medication nivolumab (Opdivo) to include the treatment of patients with metastatic squamous non-small cell lung cancer with progression on or after platinum-based chemotherapy.

Worried about potential drug shortages? There's an app for that.
Labels of prescription testosterone products must now include information about possible cardiovascular and stroke risks associated with their use.

The FDA today published several public notifications advising consumers to avoid a number of supplemental products marketed for sexual enhancement that were found to contain a potentially dangerous, undeclared ingredient.
Expanded use would include treatment of patients with advanced squamous non-small cell lung cancer who received prior therapy.
A pair of phase 3 studies recently demonstrated that Teva Pharmaceutical Industries' investigational treatment for moderate-to-severe asthma in patients with elevated blood eosinophils reduces asthma exacerbation rates by at least half.

Teva Pharmaceutical Industries' investigational drug designed to prevent chronic migraines recently demonstrated positive results in a phase 2 study.

Study finds that 61 to 82% of patients treated with experimental drug achieved undetectable viral replication levels.
A new FDA approval has given women seeking long-term birth control an additional option.
Heritage Pharmaceuticals Inc. is voluntarily recalling 10 lots of its 150 mg injectable colistimethate and 3 lots of its 600 mg injectable rifampin following FDA observations of aseptic practices at the manufacturer's site that could potentially affect the products' sterility.