
Baxter International is voluntarily recalling 2 lots of its 0.9% sodium chloride injection due to complaints of particle matter.

Baxter International is voluntarily recalling 2 lots of its 0.9% sodium chloride injection due to complaints of particle matter.
The FDA has approved the first pathogen reduction system for the preparation of plasma by blood establishments.
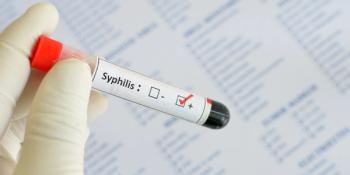
A rapid syphilis screening test will now be available in a greater number of health care settings.

The FDA will allow marketing of the EnLite Neonatal TREC Kit, a screening test for severe combined immunodeficiency in newborns.‹
According to a new study, a CCR5-blocking drug used to treat HIV may also be able to slow prostate cancer metastasis.
Preliminary evidence indicates that new treatment is both safe and effective.
Study suggests that HIV adaptations are evolving it into a weaker virus.