Ryan Marotta, Assistant Editor
Articles by Ryan Marotta, Assistant Editor

Amgen has announced positive results of a phase 3 study evaluating its investigational drug, ABP 501, a biosimilar candidate to AbbVie's fully human anti-tumor necrosis factor alpha monoclonal antibody, adalimumab, in patients with moderate-to-severe rheumatoid arthritis.

Rite Aid Corp. recently opened 24 RediClinics inside select community pharmacies situated across 2 large metropolitan markets, following the pharmacy chain's acquisition of the retail clinic operator in April 2014.

Sanofi and MannKind Corp. have announced the US launch of their Afrezza Inhalation Powder, the first and only insulin approved for administration via inhalation.

Merck has announced the US launch of its insomnia medication, suvorexant, an orexin receptor antagonist indicated for the treatment of adults who have difficulty falling or staying asleep.
Shire recently announced that the FDA has granted Fast Track designation to its investigational treatment for neurocognitive decline associated with Hunter syndrome (MPS II).
Amgen and its subsidiary, Onyx Pharmaceuticals, have submitted a supplemental New Drug Application (sNDA) to the FDA for their proteasome inhibitor, carfilzomib (Kyprolis), in the hopes of expanding the drug's indication to include patients with relapsed multiple myeloma who have received at least 1 prior therapy.

The FDA has approved a new formulation of Zogenix's extended-release hydrocodone bitartrate (Zohydro ER) with technology that provides the drug abuse-deterrent properties without changing the release properties of hydrocodone.
Hospira is voluntarily recalling 1 lot of its 0.9% Sodium Chloride Injection USP 250 mL at the user level following a confirmed report of particulate, identified by the manufacturer as human hair, in a single unit.

The FDA today approved Boehringer Ingelheim and Lilly's empagliflozin and linagliptin (Glyxambi) as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Daily fixed-dose combination tablet contains 800 mg of darunavir and 150 mg of cobicistat.

Shire's lisdexamfetamine dimesylate (Vyvanse) today became the first and only FDA-approved medication to treat binge-eating disorder in adults.

Abbott has announced the US launch of its Technis Multifocal +2.75D and +3.25D intraocular lenses (IOLs), products that the manufacturer claims will improve the vision of patients with cataracts at multiple distances.

The FDA has approved Janssen Therapeutics' darunavir and cobicistat (Prezcobix) for the treatment of human immunodeficiency virus (HIV-1) in combination with other antiretroviral agents for adults with no darunavir resistance-associated substitutions.
Purdue Pharma has announced the US commercial launch of its extended-release hydrocodone bitartrate (Hysingla ER), an opioid analgesic indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment in patients for whom alternative treatment options are inadequate.
The FDA today announced that it plans to implement a more stringent review process for automated external defibrillators (AEDs) in an effort to improve the devices' quality and reliability.
Sanofi and Regeneron Pharmaceuticals recently announced that the FDA has accepted for priority review a Biologics License Application for their investigational drug, alirocumab, which is indicated for patients with hypercholesterolemia.
The FDA has approved Rockwell Medical's Triferic, an iron compound delivered during dialysis, as an iron replacement product to maintain hemoglobin in adult patients with hemodialysis-dependent chronic kidney disease.

The FDA today approved Ivax Pharmaceuticals' esomeprazole magnesium delayed-release capsules, the first generic version of AstraZeneca's proton pump inhibitor Nexium, for the treatment of gastroesophageal reflux disease in patients aged >1 year.

The FDA has approved parathyroid hormone (Natpara), a once-daily hormonal injection to control low blood calcium levels (hypocalcemia) in patients with hypoparathyroidism.

The FDA's Anti-Infective Drugs Advisory Committee has recommended the approval of Astellas' investigational antifungal drug, isavuconazonium, for the treatment of invasive aspergillosis and mucormycosis, which are life-threatening fungal infections primarily found in immunocompromised patients.
The FDA has issued a safety alert recommending against the routine use of bone graft substitutes containing recombinant proteins or synthetic peptides among patients aged

System offers access to a large drug library, improved wireless communication, smart secondary delivery, and unique air management.

UCB recently announced that the FDA has accepted for review its New Drug Application for brivaracetam, an investigational treatment for partial-onset seizures in patients aged >16 years with epilepsy.

The FDA today approved Bexsero, a vaccine to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroup B in patients aged 10 to 25 years.
The FDA has approved Akorn's phenylephrine hydrochloride (HCl) ophthalmic solution, an alpha-1 adrenergic receptor agonist indicated to dilate the pupil.
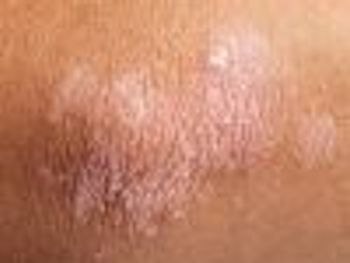
Treatment with secukinumab led to clearer skin after 12 weeks compared with placebo.
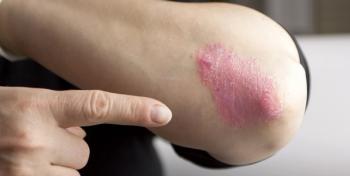
The FDA today approved Novartis' secukinumab (Cosentyx), an interleukin-17A antagonist, for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Hospira has received 510(k) regulatory clearance from the FDA for its Plum 360 medication infusion system with Hospira MedNet safety software.

Gedeon Richter and Actavis today announced positive results from a phase 3 trial evaluating the efficacy and safety of cariprazine, an investigational atypical antipsychotic, in preventing relapse among schizophrenia patients.

Janssen announced today that the FDA has granted Priority Review designation to its New Drug Application (NDA) for paliperidone palmitate, a 3-month atypical antipsychotic for treating schizophrenia in adults.





























