
Emend found to provide a greater delay in nausea and vomiting from cancer treatment than other medicines.

Emend found to provide a greater delay in nausea and vomiting from cancer treatment than other medicines.

The FDA has approved Merck's supplemental new drug application for single-dose fosaprepitant dimeglumine for injection.
Spiriva Respimat is from the first new class of inhaled medicine approved in more than 10 years of asthma treatment.

Patients with asthma can now access Spiriva Respimat with a prescription at pharmacies across the country.
Brodalumab is monoclonal antibody that targets the IL-17 receptor.

Baxter International is voluntarily recalling intravenous solution due to leaking containers and the potential for particulate matter.
The FDA is examining a biologics license application for brodalumab injection 210 mg as a treatment for moderate to severe plaque psoriasis.

Sumatriptan nasal powder is for the treatment of migraines with or without aura in adults.

The FDA has approved sumatriptan nasal powder for the treatment of migraines with or without aura in adults.

The FDA has approved Merck's elbasvir and grazoprevir with or without ribavirin for the treatment of chronic hepatitis C virus genotypes 1 and 4 infections.
Botox was initially approved for upper limb spasticity or stiffness in the elbow, wrist, and fingers in March 2010.

Once-daily, long-acting basal insulin was approved in September 2015 for improving glycemic control in adults with diabetes.

Once-daily, long-acting basal insulin was approved in September 2015 for improving glycemic control in adults with diabetes.
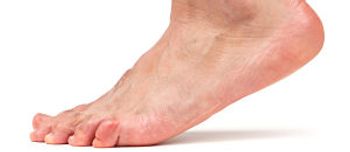
The FDA has approved onabotulinumtoxinA (Botox) as a treatment for adults' lower limb spasticity by lessening muscle stiffness in ankle and toe muscles.

Novo Nordisk's insulin degludec injection is now available in pharmacies nationwide.
Abuse-deterrent, extended-release analgesic is designed to treat chronic pain.
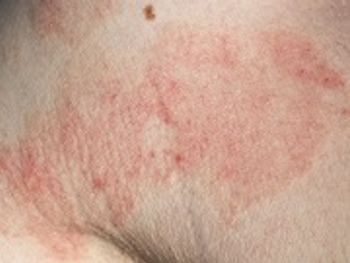
Expanded label single-dose treatment of Dalvance treats acute bacterial skin and skin structure infections caused by Gram-positive bacteria.
The FDA has approved Allergan's supplemental new drug application to update the label for dalbavancin (Dalvance).

The FDA has accepted Collegium Pharmaceutical Inc's investigational new drug application to begin a clinical trial for Hydrocodone DETERx.
Baricitinib is the only once-daily oral selective JAK1 and JAK2 inhibitor for the treatment of RA.
Baricitinib is the only once-daily oral selective JAK1 and JAK2 inhibitor for the treatment of RA.

Eli Lilly and Company and Incyte Corporation have submitted a new drug application to the FDA for the approval of oral once-daily baricitinib for the treatment of moderate to severe rheumatoid arthritis.

Advair Diskus treats patients with asthma or who need maintenance treatment of airflow obstruction.

Felbatol tablets had US sales of $53 million for the fiscal year.
After receiving approval for its abbreviated new drug application from the FDA, Mylan has launched a generic version of Meda Pharms' felbamate tablets.

Ma Ying Long Pharmaceutical Group is recalling its licorice coughing liquid, an OTC cough syrup, because of unidentified morphine.
Compounded pharmaceutical products were distributed to patients, physicians, and veterinarians in California.

Abbott's Compounding Pharmacy is voluntarily recalling all of its unexpired lots of sterile compounded products, including injectable medications, eye drops, sterile solutions, and eye ointments, out of concern for sterility assurance.
The FDA has approved Novartis' secukinumab for the treatment of 2 new indications: adults with active ankylosing spondylitis and active psoriatic arthritis.
FDA finds Kyndrisa was not ready for approval due to insufficient evidence of efficacy.