
Voluntary recall for1 lot of 5% dextrose injection USP 100/150 mL container due to potential leakage and microbial growth.

Voluntary recall for1 lot of 5% dextrose injection USP 100/150 mL container due to potential leakage and microbial growth.

B. Braun Medical Inc has initiated a voluntary recall of 1 lot of 5% dextrose injection USP 100/150 mL container due to potential leakage and microbial growth.

Lexicon Pharmaceuticals Inc has submitted a new drug application for telotristat etiprate, an oral drug for the treatment of carcinoid syndrome.

Defibrotide sodium (Defitelio) is the first FDA-approved treatment for veno-occlusive disease.
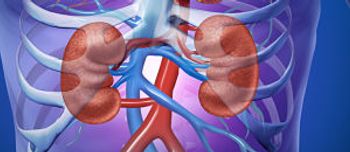
The FDA approved defibrotide sodium (Defitelio) for the treatment of hepatic veno-occlusive disease in adults and children who have additional kidney or lung abnormalities following hematopoietic stem cell transplantation.

It turns out Reliable Drug Pharmacy's products may not be so reliable after all.

Generic tramadol hydrochloride extended-release tablets treat moderate to moderately severe chronic pain.

Evista is typically given to women after menopause to treat and prevent osteoporosis and reduce the risk of invasive breast cancer.

The FDA has approved Glenmark Pharmaceuticals Inc's abbreviated new drug application for raloxifene hydrochloride tablets USP, 60 mg, which is the therapeutic equivalent to Eli Lilly and Company's Evista.
FDA seeks to ensure that generic drugs are no less abuse-deterrent than the brand-name versions on the market.
Mylan is launching tramadol hydrochloride extended-release tablets a generic version of Valeant's Ultram extended-release tablets.
The FDA has issued a draft guidance that encourages the industry's production of generic versions of opioids with abuse-deterrent formulations, while making sure they are as safe as their brand-name counterparts.

The FDA's open comment period on the return of genetic testing results will be closing March 31, 2016.

Hospira Inc, a Pfizer company, is voluntarily recalling 1 lot of 8.4% sodium bicarbonate injection due to the potential presence of particulate matter.
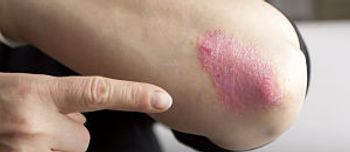
The FDA has approved Eli Lilly and Company's ixekizumab (Taltz) as a treatment for moderate to severe plaque psoriasis.
The FDA is proposing a ban on powdered gloves in most medical situations.
Yosprala is a secondary prevention for cardiovascular disease in patients at risk for aspirin-induced gastric ulcers.
Aralez Pharmaceuticals Inc resubmitted its new drug application to the FDA for its investigational candidate PA32540/PA8140 as secondary prevention of cardiovascular disease in patients at risk for aspirin-induced gastric ulcers.
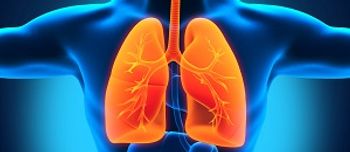
The FDA has expanded the use of crizotinib, making it the first and only FDA-approved treatment for individuals with advanced non-small cell lung cancer whose tumors have an ROS-1 gene alteration.
Evomela is the first drug approved for a high-dose conditioning indication of multiple myeloma.
Evomela is the first product to be FDA-approved for a high-dose conditioning indication for multiple myeloma.
The FDA has approved Spectrum Pharmaceuticals' melphalan for 2 new indications for patients with multiple myeloma.
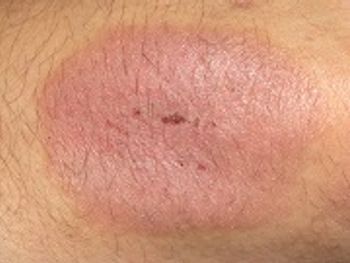
Enbrel would be the first systemic drug approved in the United States that treats chronic severe plaque psoriasis in pediatric patients.

The FDA is reviewing Amgen's supplemental biologics license application for the expanded use of etanercept to treat pediatric patients with chronic severe plaque psoriasis.

After finding glass particulate in a vial, Teva Pharmaceuticals is recalling 1 lot of its amikacin sulfate injection USP, 1 gram/4 mL, vials.
Erectile dysfunction drug expected to become available on December 11, 2017.

The FDA has approved Teva's generic version of sildenafil citrate.
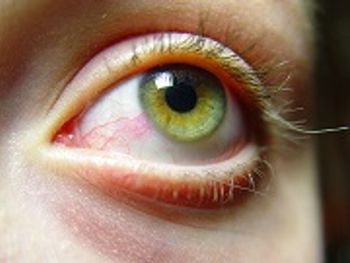
Restasis was originally approved in 2002 to treat chronic dry eye in patients.
Allergan received a complete response letter from the FDA for the manufacturer's prior approval supplement for cyclosporine ophthalmic emulsion, 0.05%, multi-dose preservative-free bottle.
Pulmicort Respules had annual sales of $217 million in the United States for 2015.