
Teva Pharmaceutical Industries Ltd has launched a new strength, 1 mg/2 mL, of the generic version of budesonide inhalation suspension.

Teva Pharmaceutical Industries Ltd has launched a new strength, 1 mg/2 mL, of the generic version of budesonide inhalation suspension.

Sagent Pharmaceuticals Inc is recalling several lots of its atracurium besylate injection due to FDA observations at the manufacturing site that call into question the products' sterility.

The FDA has approved Janssen Biotech's ibrutinib capsules for treatment-naïve patients with chronic lymphocytic leukemia.
Arymo ER treats patients with severe pain who need long-term relief and have inadequate responses to alternative treatment options.

The FDA has accepted Egalet Corporation's new drug application for morphine sulfate extended-release tablets (Arymo ER).
The application includes new data for pediatric and adolescent patients with Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).

Blincyto is currently the only FDA-approved bispecific CD19-directed CD3 T cell engager immunotherapy available for the treatment of Philadelphia chromosome-negative relapsed or refractory B-cell precursor ALL.

Amgen has submitted a supplemental biologics license application to the FDA for blinatumomab (Blincyto).

To better understand the risks of Essure, a permanent method of birth control, the FDA has called for a new mandatory clinical study to look at its risks.

The FDA has approved Roche's obinutuzumab, plus bendamustine chemotherapy, followed by Gazyva alone, as a new treatment option for patients with follicular lymphoma who did not respond to a previous regimen containing rituximab.

Allergan has received the FDA's permission to market dapsone gel, 7.5%, to patients aged 12 years and older with both inflammatory and non-inflammatory acne.

Allergan hopes to expand the label for Avycaz, in combination with metronidazole, for the treatment of complicated intra-abdominal infections.

The FDA has accepted and designated priority review to Allergan's supplemental new drug application for ceftazidime and avibactam.

The US brand and generic sales of mixed amphetamine sales capsules totaled $1.8 billion for 2015.

The FDA has approved Impax Laboratories' abbreviated new drug application for generic Adderall extended-release capsules.
Vivlodex demonstrated significant efficacy in patients with osteoarthritis pain at dose strengths of 5 mg and 10 mg.
Iroko Pharmaceuticals' meloxicam (Vivlodex) capsules, designed to treat osteoarthritis pain, are now available by prescription at pharmacies across the United States.

Injecting a patient with super-potent morphine could result in serious consequences including respiratory depression, coma, and death.

Pharmakon Pharmaceuticals has issued a voluntary recall of a morphine product that is super potent.

The FDA has approved Gilead Sciences' supplemental new drug applications for ledipasvir/sofosbuvir (Harvoni), which can treat chronic hepatitis C patients with advanced liver disease.
New drug applications for Zetia and Vytorin were for reducing the risk of cardiovascular events in patients with coronary artery disease.
Merck has received a complete response letter from the FDA regarding its supplemental new drug applications for ezetimibe and ezetimibe and simvastatin.

Rhinocort Aqua Nasal Spray with budesonide is now available OTC at all major pharmacies and retailers.

Certain lots of SyrSpend SF and SyrSpend SF Grape suspending agents recalled due to the presence of yeast.

The FDA is warning compounding pharmacies about certain lots of SyrSpend SF and SyrSpend SF Grape suspending agents due to the presence of yeast.

New drug application for Kalydeco rejected for patients with 1 of 23 residual function mutations in the cystic fibrosis transmembrane conductance regulator gene.
Vertex Pharmaceuticals has received a complete response letter from the FDA for its supplemental new drug application for ivacaftor.

The FDA has accepted for review Otsuka and Lundbeck's supplemental new drug application for a label update to brexipiprazole.
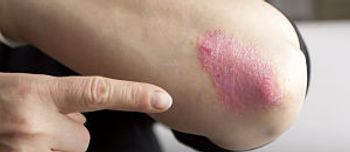
The FDA has approved betamethasone dipropionate spray, 0.05%, as a treatment for mild to moderate plaque psoriasis in patients aged 18 years and older.
Emend found to provide a greater delay in nausea and vomiting from cancer treatment than other medicines.