
FDA Approves Birch Triterpenes Topical Gel for Treatment for Adult, Pediatric Patients With JEB, DEB
This approval makes birch triterpenes the first treatment for wounds associated with junctional epidermolysis bullosa to be approved by the FDA.

Gillian McGovern is an editor at Pharmacy Times®. She graduated from Rowan University in 2023 with a BA in Writing Arts and concentrations in Publishing & Writing for the Public, Technical & Professional Writing, and Creative Writing.

This approval makes birch triterpenes the first treatment for wounds associated with junctional epidermolysis bullosa to be approved by the FDA.

The study authors note that interventions to reverse frailty, such as screening and assessments to identify issues, can help in the management of COPD and asthma and lead to better prognoses.
Previously, garadacimab was granted an orphan drug designation as a therapy for hereditary angioedema by both the FDA and EMA.

Treatment options can be limited for this patient population.

Using information from clinical studies and her experiences working in the field, Stephanie Kirk describes treatment options that are on the horizon, and how removing stigma is key in HIV care.
The study, which consists of 3 trials, will continue with oral PrEP regimens; however, the vaccination trials have stopped due to the unlikely chance of the vaccine showing efficacy.
The differences between the regimens, anti-PD1/CTLA-4 and anti-PD1/LAG-3, can be used to optimize outcomes for patients with melanoma, particularly in those who develop drug resistance.
The protein STAT3 is present in approximately 70% of tumors, with elevated levels of the protein being an indicator of poor prognoses in patients.

This is the first FDA approval for a therapy intended to reduce the risk of relapse in patients with high-risk neuroblastoma.

Both individual and structural barriers were the most common hindrances to PrEP roll-out, and the investigators emphasize that efforts to remove the obstacles as well as further research can help improve PrEP implementation.

The study authors note that low skin cancer screening (SCS) participation, the participants’ better health than nonparticipants, and examination biases may be contributing factors to the insignificant results.
Compared to placebo plus chemotherapy, quizartinib plus chemotherapy improved OS without significant AEs and impact on QOL in patients with AML.
The authors note that CAR-T cell therapy could change the treatment standard for patients with DLBCL, who previously had few options if standard chemotherapy treatment was ineffective.

The study results demonstrated that 92% of patients treated with benralizumab were able to safely reduce inhaled steroid dose, and more than 60% could stop use entirely.

The study authors note that the Best ACT-S questionnaire complements the Best ACT-P questionnaire, a previously developed questionnaire that assesses preschool-aged patients with asthma.

Study results indicate insignificant differences between PFS and OS in patients with HR[+]/HER2[-] breast cancer who received either palbociclib-letrozole or palbociclib-fulvestrant treatment.

This finding can be a first step toward getting more infants to HIV remission, allowing them to remain off antiretroviral drugs for longer periods.

This FDA approval represents the first cell-based gene therapies for the treatment of sickle cell disease in patients 12 years of age and older.
The study authors note that stress conditions result in an increased activity of the protein HDAC8, which promotes melanoma cell survival.

The study authors note that addressing environmental factors, such as IPV and food insecurity, can provide further benefits in neurodevelopment regardless of maternal HIV status.

During clinical trials, individuals treated with iptacopan had increased hemoglobin levels and did not need to receive blood transfusions.
Patients with breast cancer who underwent chemotherapy and skipped RNI did not face an increased risk of disease recurrence or death 5 years post-surgery.
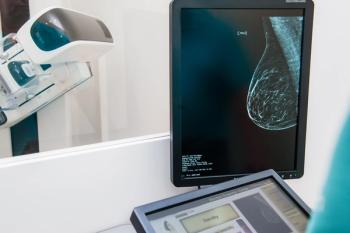
Although PFS was improved in patients with unresectable locally advanced or metastatic HER2-positive breast cancer, OS data were immature, requiring further research.
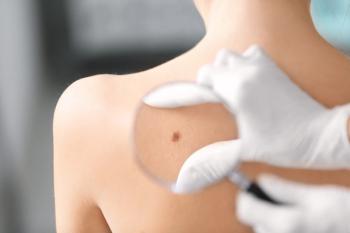
Patients enrolled in the study had substantial absolute risks and elevated relative risks, suggesting that AKs may be a clinical marker of UV exposure and increased skin cancer risk.

In the first-line setting, inavolisib plus palbociclib and fulvestrant improved PFS in patients with HR-positive breast cancers; however, further research is needed to understand the trends in OS.

The study authors note that increasing education, implementing online services, and removing stigma can help improve the uptake of PrEP in this population.

Currently, there is no established standard of care treatment approach of HGBL, with frontline interventions consisting of chemo-immunotherapies.

The study authors note that additional policies to reduce structural stigma against sexual minorities could continue to improve the rate of individuals who initiate PrEP use.

Pirtobrutinib is the first and only non-covalent BTK inhibitor to be approved by the FDA and is indicated for adult patients who already received at least 2 prior lines of therapy.
Further research of these mechanisms could be significant in developing future strategies to disrupt the viral persistence in individuals living with HIV.