
Widespread COVID-19 booster shot administration is pending FDA approval and recommendation by the Advisory Committee on Immunization Practices.

Aislinn Antrim is managing editor at Pharmacy Times®. She graduated from the University of North Carolina at Chapel Hill in 2019 and received her master’s in communication and media at Rutgers University in 2023.

Widespread COVID-19 booster shot administration is pending FDA approval and recommendation by the Advisory Committee on Immunization Practices.

Amesh Adalja, MD, FIDSA, FACP, FACEP, said that the efficacy of COVID-19 booster vaccines against variants of concern is still relatively unknown, although administering the third shots does increase antibodies as expected.

Specific infection-fighting cells of the immune system found to be downregulated, offering a potential clue to the cause of prolonged COVID-19 illness in children.
An announcement from CDC officials recommending COVID-19 boosters for all adults could come as soon as this week, although doses would only be widely administered once the FDA formally approved the vaccines.
Savings for these medications range from 40% to 75.8%, study results show.
The expanded indication follows Lyumjev’s original approval in 2020 and offers an important new delivery mechanism for patients with diabetes.

The approval marks the first HIF-2α inhibitor therapy approved in the United States for some types of Von Hippel-Lindau disease-associated tumors.
Experts anticipate a return to high influenza rates as COVID-19 prevention measures decline.
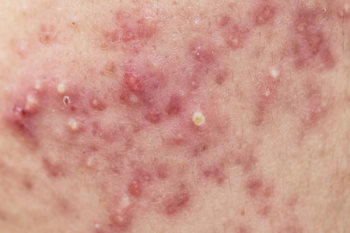
Trial is the first to suggest that targeting interleukin-4 and interleukin-13 can help address symptoms such as persistent itching and hives.

The application is based on overall response data from 90 pooled patients in the KEYNOTE-158 study who received pembrolizumab monotherapy.

The CDC now recommends COVID-19 vaccination for all individuals 12 years and older in the United States, including those who are pregnant or breastfeeding.

If approved, pembrolizumab would be the first adjuvant immunotherapy option for this patient population with renal cell carcinoma.
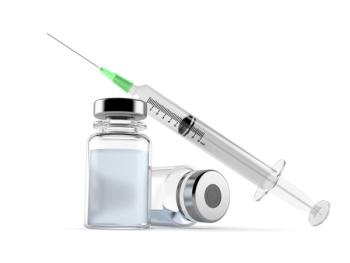
Study suggests that immediate onset mRNA vaccine reactions may not be caused by classic allergy, or IgE-mediated hypersensitivity.

The strongest associations with attention-deficit hyperactivity disorder were present for nervous system, respiratory, musculoskeletal, and metabolic diseases.

Polatuzumab vedotin regimen is the first in 2 decades to show such significant improvements.

Avalglucosidase alfa-ngpt is indicated for patients aged 1 year and older with late-onset Pompe disease.

A stepwise approach to treatment for multisystem inflammatory syndrome, beginning with initiation of immunomodulatory drugs, was successful in all patients included in the study.

Findings reflect fewer visits to health facilities, social distancing, and other safety measures implemented during the pandemic.
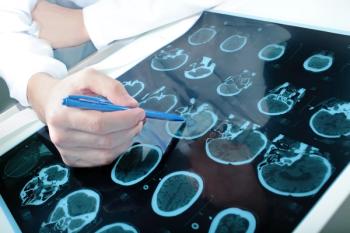
Donanemab induced rapid amyloid plaque reduction at 24 weeks in patients with early symptomatic Alzheimer disease, with the most rapid clearance in patients with the most severe plaque burden at baseline.

Anifrolumab is a first-in-class type I interferon receptor antibody and is the only new treatment in more than a decade for this patient population.

Complications of varicella-zoster virus in patients with lupus may be attributable to immunological abnormalities, lymphopenia conditions, and immunosuppressive therapies.

Investigators estimated that globally, 4.1% of all new cancer diagnoses in 2020 were attributable to alcohol consumption.

Women are significantly less likely than men to undergo coronary artery bypass grafting because of gender disparities in the understanding and treatment of coronary artery disease, research shows.

A decrease in cancer-associated macrophage-like cells was associated with an approximately 300% increase in mean progression-free survival.
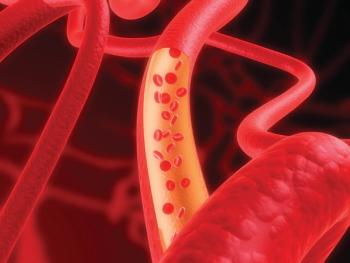
Based on their findings, investigators said modifiable risk factors are strongly associated with C-reactive protein levels in patients with coronary artery disease.

Individuals with attention-deficit hyperactivity disorder and other diagnoses who are taking stimulant medications are also more likely to switch to non-stimulant medications.
Investigators hypothesize that concurrent symptoms of anxiety and depression may be linked to concerns about adverse events.
The clinical trial expansion is part of efforts to detect potential adverse effects in children, such as heart inflammation problems.

The combination of pembrolizumab and chemotherapy marks the first immunotherapy regimen approved for patients with high-risk early-stage triple-negative breast cancer.

Two of the studies also found that patients with COVID-19 who received remdesivir had a significantly increased chance of discharge from the hospital by day 28.