
Guarding against external air pollution while reducing the risk of infection when driving presents a challenge.

Aislinn Antrim is managing editor at Pharmacy Times®. She graduated from the University of North Carolina at Chapel Hill in 2019 and received her master’s in communication and media at Rutgers University in 2023.

Guarding against external air pollution while reducing the risk of infection when driving presents a challenge.
The nasal spray bypasses the gut and potential absorption issues associated with it, and offers consistent relief even when administered hours after the onset of a migraine attack.
The guidance noted it is preferable for immunocompromised patients to receive their third dose in a health care delivery setting rather than a pharmacy or public vaccination clinic.

Calls to poison control centers regarding the use of ivermectin for the prevention or treatment of COVID-19 have increased 5-fold since its baseline before the COVID-19 pandemic.

Atezolizumab was granted accelerated approval for the mTNBC indication in March 2019, making it the first immunotherapy agent to be approved in this setting.
The FDA has also converted the indication for pembrolizumab from an accelerated to a full, regular approval for patients with urothelial carcinoma.

Investigators found that 65% of patients with cancer and COVID-19 were hospitalized and 17% required admission or transfer to a higher level of care.

Although the average demand for tocilizumab increased by 279% during the study period, fill rates for orders by hospitals dropped from 99% to 45%.
In addition to early screening, counseling patients on preventive interventions can reduce the risk of developing prediabetes or type 2 diabetes.

This is the first time that the intravenous (IV) formulation of brivaracetam will be available for pediatric patients and is the only IV formulation approved for partial-onset seizures and this age group in nearly 7 years.

Silmitasertib has produced clinical benefit as a monotherapy and in combination with drugs such as gemcitabine and cisplatin.

The approval of Tibsovo marks the first and only targeted therapy approved for patients with previously treated IDH1-mutated cholangiocarcinoma.
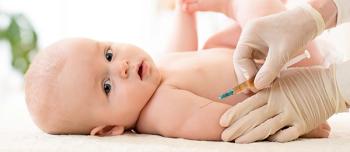
At 30 days following the third dose in the series, the pneumococcal 15-valent conjugate vaccine was non-inferior to the 13-valent vaccine for all 13 shared serotypes.

Recent research has linked gut microbiota and cardiovascular disease with reported differences in gut microbial compositions among individuals with and without cardiovascular disease.

Tisagenlecleucel is a CD19-directed genetically modified autologous T cell immunotherapy already indicated for B-cell precursor acute lymphoblastic leukemia and diffuse large B-cell lymphoma.

Between 40% and 50% of patients respond long term without relapsing.

SARS-CoV-2 neutralizing titers against the wild-type strain one month after the booster dose were 3.3 times higher than the titers found one month after the second dose.

A final blinded analysis of data from the phase 3 COVE study found 93% efficacy of Moderna’s COVID-19 vaccine, which remained durable through 6 months after the second dose.
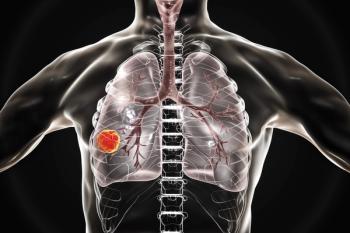
Researchers found that the levels of serum albumin and the number of metastatic sites that patients with lung cancer have were significantly associated with overall survival.

Although not all children are eligible for vaccination, recommendations for schools include masking, testing, and quarantine policies, as well as vaccinations for anyone eligible.

Rivaroxaban is the first and only therapy indicated for both coronary artery disease and peripheral artery disease.
Hospitalizations related to C. diff infections can cost more than twice as much as the average hospitalization and can extend patients’ stays by an average of 3.6 days.

Walgreens also announced expanded age eligibility for flu shots, with pharmacy team members able to vaccinate patients 3 years of age and older.
Rylaze was granted Fast Track Designation by the FDA in October 2019 for acute lymphoblastic leukemia and was approved as part of the Real-Time Oncology Review program.

Washington state's House Bill 5203 allows health care authority to partner with other agencies to produce, distribute, and purchase the drugs.
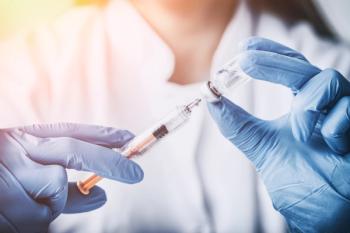
The first fully approved vaccine for COVID-19 will be marketed under the name Comirnaty.

Median disease-free survival was nearly twice as long among patients with urothelial carcinoma who received nivolumab compared with placebo.

STRO-002 is a folate receptor alpha-targeting antibody drug conjugate for the treatment of patients with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer who have received 1 to 3 prior lines of systemic therapy.

Study indicates that the genetics affecting aggression in children with attention-deficit hyperactivity disorder and disruptive behavior disorders are the same as genetics underlying aggression in the general population.

In clinical trials, empagliflozin significantly reduced the relative risk of the primary composite endpoint of time to cardiovascular death or hospitalization for heart failure by 25%.