
Molnupiravir reduced the risk of hospitalization or death by approximately 50% among patients with COVID-19 who received the drug.

Aislinn Antrim is managing editor at Pharmacy Times®. She graduated from the University of North Carolina at Chapel Hill in 2019 and received her master’s in communication and media at Rutgers University in 2023.

Molnupiravir reduced the risk of hospitalization or death by approximately 50% among patients with COVID-19 who received the drug.

Among 38 patients with B-cell derived malignancies who were seronegative after 2 COVID-19 vaccine doses, 55% had detectable antibodies after the third dose whereas 45% remained seronegative.
The recommendation for managing cytopenias among patients treated with tyrosine kinase inhibitors is to discontinue treatment at the onset of grade 3 or higher.

The combination of cetuximab and encorafenib resulted in a median overall survival of 8.4 months, compared to 5.4 months in the control arm among patients with colorectal cancer.

Significant reductions in mean monthly migraine days compared with placebo were seen as early as weeks 1 through 4 in the clinical trial program for atogepant.

Risk stratification for survival should not dictate treatment, but stratification for thrombosis risk should play an important role in treatment decisions.

Durvalumab in combination with oleclumab reduced the risk of non-small cell lung cancer disease progression or death by 56%, whereas patients receiving durvalumab and monalizumab had a 35% reduction.

Monthly treatment with evolocumab reduced LDL-C by a mean of 38% from baseline compared to placebo among patients ages 10 to 17 with heterozygous familial hypercholesterolemia.

Policy makers should consider structural incentives to improve generic drug access, investigators say.

Co-administration of COVID-19 vaccines and flu vaccines is also available.
The prostate-specific membrane antigen-targeted positron emission tomography imaging technique will be part of the National Comprehensive Cancer Network guidelines beginning in 2022.

The COVID-19 booster doses are to be administered at least 6 months after completion of the primary 2-dose series and are the same formulation and dosage strength as the original doses.
Remdesivir demonstrated a statistically significant 87% reduction in risk for the composite primary endpoint of COVID-19-related hospitalization or all-cause death by day 28.
Patients with triple-negative breast cancer who received sacituzumab govitecan had a median progression-free survival of 4.6 months compared to 2.3 months with chemotherapy.

Trial results showed a 24% confirmed objective response rate with a median duration of response of 8.3 months among patients with cervical cancer who received tisotumab vedotin-tftv.
Relatlimab is the first LAG-3-blocking antibody to demonstrate a clinical benefit for patients with melanoma in phase 3 data.

The results are the first from a pivotal trial of any COVID-19 vaccine in children under 12 years of age, with results in children under 5 years of age expected as soon as later this year.

The panel did, however, vote to recommend the booster shots to adults over 65 years of age and those at high risk of severe COVID-19.

Strong immune memory lasted in all age groups tested after receiving the Moderna vaccine, including individuals over 70 years of age who are especially vulnerable to COVID-19.
Clinical trial results found a 30% reduction in the risk of death and a 2.3-month extension in median overall survival among patients with advanced or metastatic esophageal squamous cell carcinoma treated with tislelizumab.

Survey participants saw potential for immunotherapy use in earlier stages of several diseases, including melanoma, lung cancer, and bladder or urothelial cancer.

In the MAGNOLIA trial, the median duration of response was not reached at the median follow-up time of 8.3 months, with 85% of responders still in remission at 12 months.
Among patients treated with the combination of nivolumab and ipilimumab, 23% were alive at 3 years, compared to 15% of patients treated with chemotherapy.
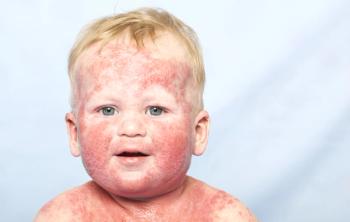
Improvements in moderate-to-severe atopic dermatitis began quickly after the first dose of dupilumab, with improved itching in 1 week and skin clearance in 2 weeks.
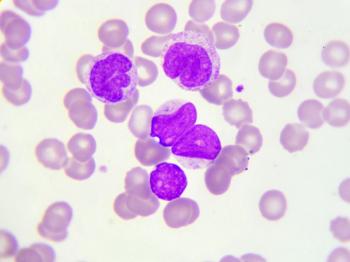
With CAR T-cell therapies showing promise for follicular lymphoma and marginal zone lymphoma, clinicians must weigh toxicity and tolerability almost as strongly as efficacy because patients can live years with these diseases

Six-point plan outlines strategies to increase national vaccination rates, keep schools open, increase testing and masking access, and to relieve health systems that are overrun with COVID-19 patients.
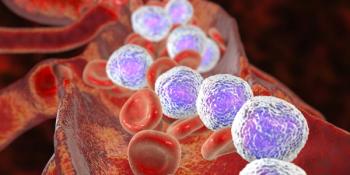
The efficacy of pirtobrutinib does not depend on prior therapy, reason for prior BTK inhibitor discontinuation, or C481 mutation status, according to the presentation.

Empagliflozin demonstrated a 21% relative risk reduction for the composite primary endpoint of cardiovascular death or hospitalization for heart failure, compared with placebo.

Nearly 20% of patients with multiple myeloma have a form of the disease in which they make high quantities of a component of monoclonal proteins, which damages the kidneys.

In a study, 92.5% of patients treated with the 6-month regimen of Invega and 95% of patients treated with the 3-month regimen were relapse-free at 12 months.