Commentary
Article
Proper Use of Automation in Hazardous Drug Compounding Pharmacies
Although robotics can be instrumental in assuring sterility and reducing the overall number of potential hazardous drug exposure events, certain tasks require vigilant oversight.
Introduction
Pharmacy cleanrooms have come to rely on automation to improve product preparation safety, patient safety workflow efficiency, and worker protection. Automation and technology in a cleanroom can take the form of compounding devices, robotics, intravenous workflow management systems (IVWMS), barcode scanning, gravimetrics, and more.
With the recent November 2023 enforcement date for USP compounding standards, hospital pharmacies are increasingly turning to automation to ensure safety in the compounding process. Although the use of robotics can be instrumental in assuring sterility and reducing the overall number of potential hazardous drug (HD) exposure events in a compounding pharmacy, certain tasks that involve automation or robotics and the handling of HDs require vigilant oversight.
Image credit: luchschenF | stock.adobe.com
The implementation of automation in compounding pharmacies has led to industry wins. It has also raised “watchpoints” that need to be prudently evaluated and addressed with the use of robotics and automation to accurately assess HD exposure potential and ensure the safety of pharmacists and pharmacy technicians.
Wins
The need to implement sterile compounding automation has become more widely recognized since the 2013 publication of the ISMP Guidelines for Safe Preparation of Compounded Sterile Preparations.1 Key operational wins to support this implementation include operational efficiency, product sterility, and personnel safety.
Facilities recognize the need for compounding automation and are expected to maintain or grow their pharmacy automation budgets over the next year, according to the 2023 State of Pharmacy Automation Survey data.2 Among small and medium-size hospitals, only a very small percentage of facilities have adopted compounding robots, while almost a quarter of larger hospitals (400+ beds) have implemented compounding robots. IVWMS have increasingly become more prevalent in medium-size and large hospital cleanrooms (100–400+ beds), with over a third of facilities planning to implement these tools. Like the adoption of compounding robots, a lower percentage of facilities (100 beds or fewer) plan to implement IV workflow technology.2
One organization that works to increase the adoption of IV automation safety practices is THRIV, a coalition for IV accuracy. THRIV “champions the universal adoption and faithful utilization of IVWMS in health-system pharmacies.”3 Protection of the product and patient must serve as the key tenets of sterile compounding staff and pharmacy leadership. THRIV, in collaboration with the Allegheny Health Network pharmacy leaders and administrative residents, has published and maintains the IV-WMS/THRIV Bibliography. This tool helps pharmacy leaders find data to support the approval of resources for the implementation of sterile compounding technology and automation.4
The 2022 ISMP Guidelines for Sterile Compounding and the Safe Use of Sterile compounding Technology outline specific recommendations, best practices, and safety gaps with automated compounding devices, IVWMS, and IV robots.5 This document is an asset to pharmacy leaders and other administrators in identifying and justifying the need for pharmacy compounding technology.
The benefits of compounding automation and technology for product preparation and their impact on three key factors—patient safety, accuracy, and sterility—are well established.6 A fourth and very important justification for sterile compounding technology and, specifically, the need for robotics in HD compounding as an engineering control pertains to worker protection.
When considering personnel safety in HD compounding pharmacies, compounding robotics reduce workers’ direct interaction with HDs and related exposure potential. They also reduce the potential for ergonomic and repetitive motion injuries associated with manual compounding.7
When making the case for pharmacy automation and technology in HD compounding, consider how personnel protection and reduced risk of potential HD exposure will be implemented. Most institutions that compound HDs have worked over the past decade to implement Closed System Drug-Transfer Devices (CSTDs). It is widely recognized that CSTDs have the ability to decrease HD exposure, and more than 90% of institutions surveyed use CSTDs in the pharmacy for HD preparation, while close to 80% of drug administration in clinical areas have implemented CSTDs.2
Areas to Watch
These wins are significant for product, personnel, and patient safety. However, the potential for exposure can be greater due to the sheer volume of HDs in the operation, especially in high volume HD compounding facilities. As institutions move forward with assessing and implementing HD compounding automation, they must take a risk-assessment approach for the whole cycle of the automation operation and related maintenance and troubleshooting activities.
Several key areas regarding the routine operation of HD robotics to reduce potential exposure require a careful and detailed risk-assessment approach (see Figure1).
Figure 1. Robotic cycle of operation and related watchpoints for potential HD exposure.1
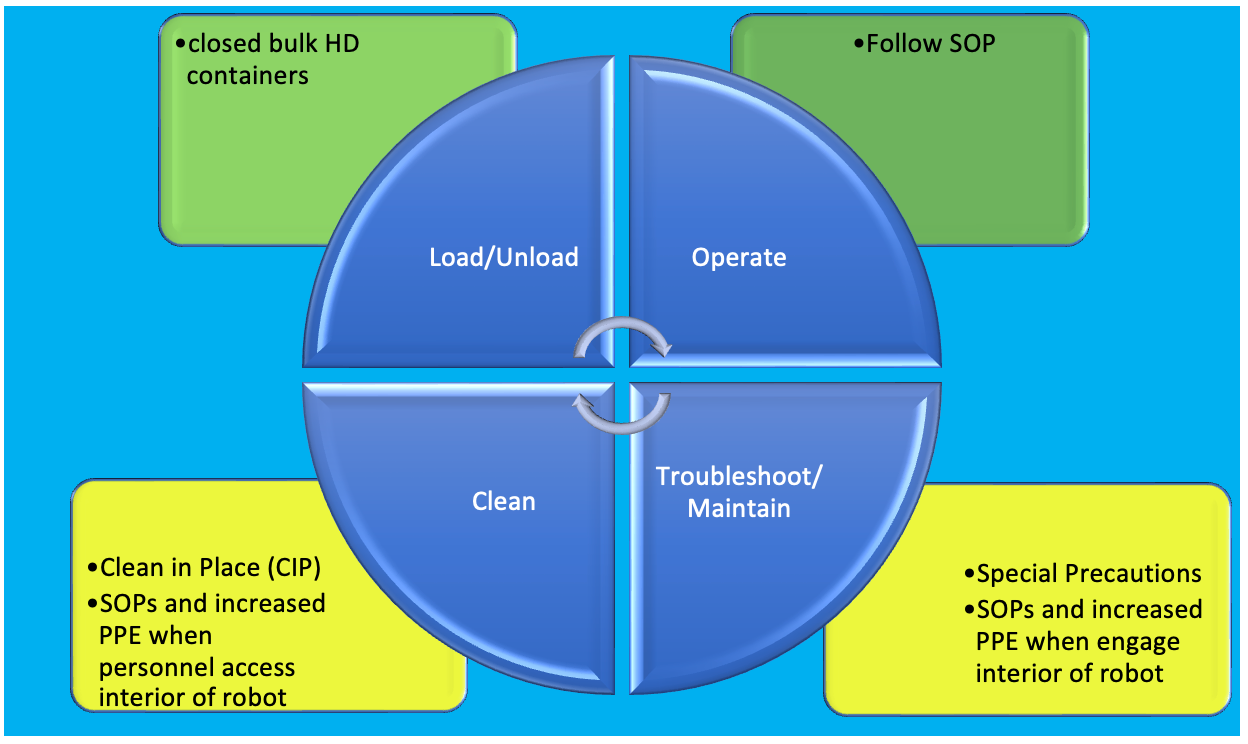
- Loading of bulk HDs:
- This should be a well-engineered system where personnel interactions are limited to the placement and handling of closed bulk HD containers.
- Troubleshooting mechanical or automation issues and direct intervention with automation equipment:
- Increased efficiency can result in increased risk of exposure for operators working under time pressure to resolve mechanical issues and quickly restart robotic equipment.
- Residual HD may be present on surfaces and/or inadvertently aerosolized into the operator’s breathing zone.
- Standard operating procedures (SOPs) are critical for troubleshooting activities and must be paired with increased levels of personal protective equipment (PPE) for operators.
- Automation equipment cleaning and maintenance
- Cleaning and maintenance may require personnel to directly interact with contaminated surfaces in small interior environments.
- HDs on surfaces may be touched and can also be re-aerosolized.
- Best practice will include fully engineered clean-in-place (CIP) systems.
- Cleaning and maintenance SOPs and increased levels of PPE for operators are critical.
In HD compounding, robotics automation can protect the product, patient, and personnel. Pharmacy leadership and administrators should investigate and take the time to identify HD exposure risk points for personnel operating HD robotics.
These issues have been identified and noted in the AIHA Hazardous Drug Surface Contamination Guidance Document (see Table).8
Table8
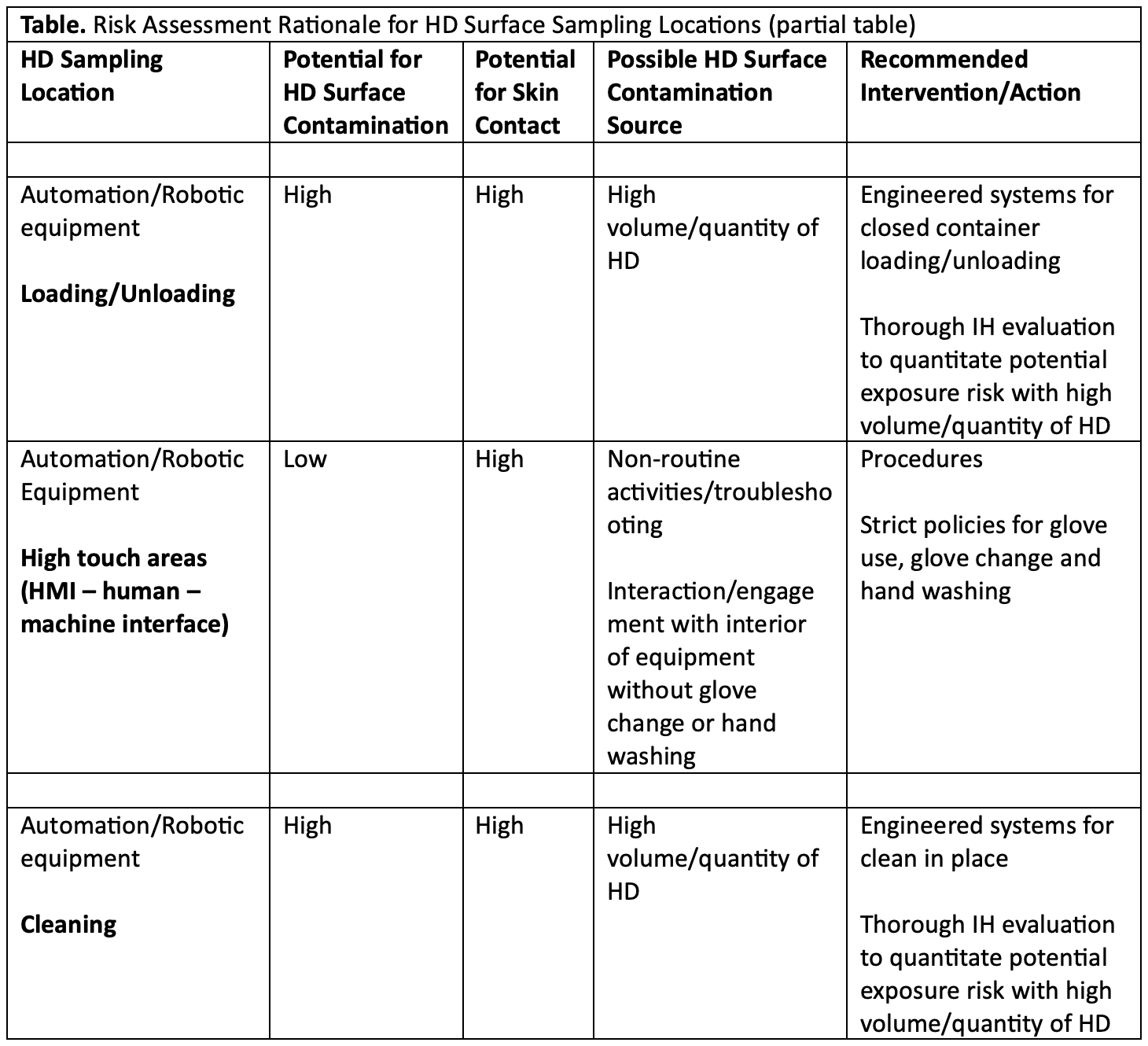
In the assessment of risk, it is important to consider not just the potential surface contamination but also the potential aerosolization of HDs and to establish robust sampling strategies for both air and surface. These confirmation sampling activities can follow the International Society for Pharmaceutical Engineering (ISPE) guidance for containment verification.9 This is the best-practice standard used in the pharma/biopharma industry to control effectiveness automation and engineering solutions provide and to very accurately measure the potential for operator exposure to HDs (or active pharmaceutical ingredients [API] with surrogates).
Solutions
As you implement robotics in your compounding pharmacy, follow these steps for effective protection during the full operation life cycle:
- Implement the Hierarchy of Controls for operations, maintenance, and cleaning.10
- Conduct a thorough assessment of risk that includes operations, maintenance, and cleaning.
- Follow the pharma/biopharma industry model with the ISPE good practice guide with surrogates to evaluate potential for exposure to surface and aerosol contamination during operations, maintenance, and cleaning steps. Lean into lessons learned in the pharma/biopharma industry with similar principles and activities on a smaller scale.Occupational exposure limits and acceptable surface limits are available and can be used to establish safe levels of each HD in the air and on surfaces. Surrogate testing with very sensitive detection limits can effectively be used to evaluate robotics and related operations and interventions prior to the introduction of HDs.
- Build robust SOPs for operations, troubleshooting, maintenance, and cleaning.
- Use higher levels of PPE as needed for higher-risk activities.
- Maintain accountability as a team as you implement robotics engaging all parties in planning, construction, operation, and maintenance. Team members include compounding pharmacy staff members, pharmacy managers, facility and system engineers, and health and safety professionals.
About the Authors
Amy Snow, MHS, CIH, CSP, is Managing Consultant with Trinity Consultants | SafeBridge.
Elaine Strauss, PharmD, MS, BCSCP, is Senior Consultant of Cleanrooms and Sterile Compounding with WorkingBuildings, a Trinity Consultants Company.
References
1. Institute for Safe Medication Practices (ISMP). ISMP guidelines for safe preparation of compounded sterile preparations. 2013, revised 2016. https://www.ismp.org/sites/default/files/attachments/2017-11/Guidelines%20for%20Safe%20Preparation%20of%20Compounded%20Sterile%20Preperations_%20revised%202016.pdf. Accessed Nov. 28, 2023.
2. Survey respondents. State of Pharmacy Automation. 2023;20(8):6. https://www.pppmag.com/article/3127. Accessed Nov. 28, 2023.
3. THRIV Coalition for IV Accuracy. Why THRIV? Our mission. https://www.thrivcoalition.org/our-mission/. Accessed Nov. 28, 2023.
4. THRIV Coalition for IV Accuracy. The IVWMS/THRIV Bibliography. https://www.thrivcoalition.org/iv-workflow-bibliography/. Accessed Nov. 28, 2023.
5. Institute for Safe Medication Practices (ISMP). ISMP guidelines for sterile compounding and the safe use of sterile compounding technology. May 4, 2022. https://www.ismp.org/resources/guidelines-sterile-compounding-and-safe-use-sterile-compounding-technology. Accessed Nov. 28, 2023.
6. Boyce CA, Erickson BA. Sterile compounding technology. In: Forrey RA, Amerine LB, Yaniv AW. Sterile Compounding Preparations. 5th ed. Bethesda, MD: American Society of Health-System Pharmacists; 2023.
7. Forrey RA, Amerine LB, Yaniv AW. Sterile Compounding Preparations. 5th ed. Bethesda, MD: American Society of Health-System Pharmacists; 2023.
8. American Industrial Hygiene Association (AIHA). Hazardous drug surface contamination. Table 5.1: Risk assessment rationale for HD surface sampling locations. 2003; 37-39. https://aiha-assets.sfo2.digitaloceanspaces.com/AIHA/resources/Guidance-Documents/Hazardous-Drug-Surface-Contamination-Guidance-Document.pdf. Accessed Nov. 28, 2023.
9. International Society for Pharmaceutical Engineering. Good Practice Guide: Assessing Particulate Containment Performance of Pharmaceutical Equipment. 2nd ed. May 2012. https://ispe.org/publications/guidance-documents/assessing-particulate-containment-performance
10. AIHA Hazardous drug surface contamination, Figure 4.3: Hierarchy of controls (NIOSH). 2003; 30. https://aiha-assets.sfo2.digitaloceanspaces.com/AIHA/resources/Guidance-Documents/Hazardous-Drug-Surface-Contamination-Guidance-Document.pdf. Accessed Nov. 28, 2023.
Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.






