Précis
Integration of these agents requires supportive care considerations for cytokine release syndrome, neurotoxicity, infection, and antigen-specific toxicities to ensure optimal care of patients with multiple myeloma receiving T-cell–engaging therapies.
Abstract
The treatment of patients with relapsed/refractory (R/R) multiple myeloma (MM), particularly those with triple-class refractory MM, poses a clinical challenge due to the low overall response rates and historically poor prognosis with previously available therapies. Agents with novel mechanisms of action, such as anti–B-cell maturation antigen (BCMA) chimeric antigen receptor T cells and, more recently, anti-BCMA bispecific T-cell engagers (TCEs), have demonstrated promising activity for most of these patients. In this review, we aim to summarize the current landscape of TCEs for the treatment of patients with R/R MM, focusing not only on approved agents, such as teclistamab-cqyv (Tecvayli; Janssen Biotech, Inc), elranatamab-bcmm (Elrexfio; Pfizer Inc), and talquetamab-tgvs (Talvey; Janssen Biotech, Inc), but also on supportive care strategies intended to inform clinicians on how to integrate these innovative therapies into patient care. Given available evidence, the use of TCEs in MM must include a comprehensive approach to the monitoring, prophylaxis, and treatment of infections and to the supportive care for potential adverse events, such as cytokine release syndrome and neurotoxicity, including immune effector cell–associated neurotoxicity syndrome, and antigen-specific on-target, off-tumor toxic effects. The advent of TCEs marks a pivotal shift in the treatment of patients with R/R MM and underscores the role of health care providers, particularly pharmacists, in the management and mitigation of adverse events associated with TCE therapy to optimize patient outcomes within this evolving treatment landscape.
Introduction
Multiple myeloma (MM) is a complex hematologic malignancy characterized by the clonal proliferation of plasma cells and their interplay within the tumor microenvironment.1,2 The landscape of MM therapeutics has witnessed a paradigm shift with the advent of the T-cell engagers (TCEs) teclistamab-cqyv (Tecvayli; Janssen Biotech, Inc), elranatamab-bcmm (Elrexfio; Pfizer Inc), and talquetamab-tgvs (Talvey; Janssen Biotech, Inc).3-5 Although TCEs can achieve high response rates in patients with relapsed/refractory (R/R) MM, optimal use of supportive care is necessary for cytokine release syndrome (CRS) and neurotoxicity, including immune effector cell–associated neurotoxicity syndrome (ICANS).6 Furthermore, with the high incidence of severe infections, particularly opportunistic infections such as Pneumocystis jirovecii pneumonia (PJP) and cytomegalovirus (CMV), monitoring, prophylaxis, and prompt treatment for infection are necessary to mitigate infection-related morbidity and mortality.7,8 It is also important to optimize support measures for the on-target, off-tumor toxic effects (ie, oral and nail toxicities) of talquetamab. This review aims to summarize the pivotal data that led to the accelerated approval of these TCEs as well as important considerations for the use of these therapies. These considerations include monitoring and management of CRS, neurotoxicity, and infection and tailored supportive care for select toxicities with talquetamab.
Teclistamab, Elranatamab, and Talquetamab
Teclistamab and elranatamab are bispecific B-cell maturation antigen (BCMA)–directed T-cell engaging antibodies that bind BCMA on plasma cells, plasmablasts, and MM cells and CD3 on T cells, leading to cytolysis of the BCMA-expressing cells.4,5 Talquetamab is a bispecific antibody that targets CD3 on T cells and G protein–coupled receptor family C group 5 member D (GPRC5D).3 Although GPRC5D exhibits heightened expression in malignant plasma cells vs normal plasma cells, it is pertinent to note that the expression of GPRC5D is predominantly confined to malignant plasma cells. However, trace expression GPRC5D is detected in the skin and testes, possibly contributing to unintended on-target, off-tumor toxic effects.9
The trials that led to the accelerated approvals based on response rates of teclistamab, elranatamab, and talquetamab were MajesTEC-1 (NCT04557098), MagnetisMM-3 (NCT04649359), and MonumenTAL-1 (NCT03399799), respectively.3-5 Their FDA-approved indication is in adult patients with R/R MM who have previously received at least 4 lines of therapy, including a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), and an anti-CD38 monoclonal antibody (Table 1).10-12
Teclistamab
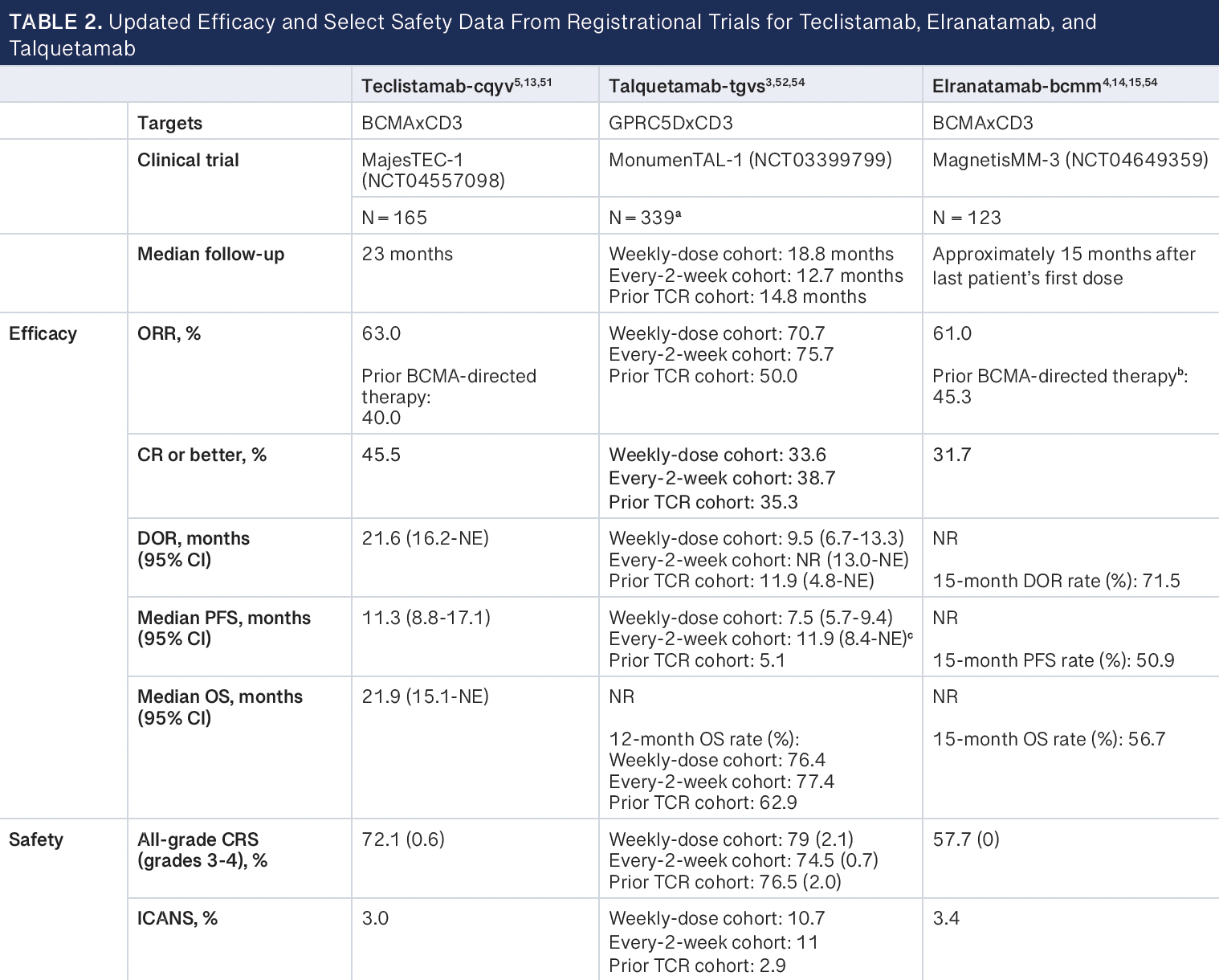
The MajesTEC-1 phase 2 trial assessed the efficacy and safety of teclistamab in adult patients with R/R MM who had received at least 3 prior lines of therapy, including an IMiD, a PI, and an anti-CD38 antibody.5 The study enrolled 165 patients, with a median follow-up of 14.1 months at the data cutoff of March 16, 2022, with a median duration on treatment of 8.5 months (range, 0.2-24.4). The patient population exhibited high resistance to previous therapies, and a substantial proportion had triple-class and penta-drug refractory disease. The overall response rate (ORR) was 63%, with 39.4% of patients achieving a complete response (CR) or better. The median time to first response was 1.2 months, and negativity for minimal residual disease (MRD) at any time point was reported in 81.5% of MRD-evaluable patients.13 Certain factors, including extramedullary disease, International Staging System stage III disease, and extensive bone marrow involvement, correlated with lower response rates. Notably, patients with fewer previous lines of therapy demonstrated higher response rates. Responses were sustained, with a median duration of 18.4 months (95% CI, 14.9-not estimable). The median progression-free survival was 11.3 months (95% CI, 8.8-17.1), with a median overall survival of 18.3 months (95% CI, 15.1-not reached [NR]).13
Results from extended follow-up of approximately 2 years with a data cutoff date of January 4, 2023, have been reported (Table 2).
Elranatamab
The phase 2 MagnetisMM-3 clinical trial evaluated the efficacy and safety of elranatamab and included patients with R/R MM who were refractory to at least 1 PI, 1 IMiD, and 1 anti-CD38 antibody and relapsed or refractory to their last antimyeloma regimen.4 Of the 187 patients enrolled, 123 patients were in cohort A (no prior BCMA-directed therapy) and 64 patients were in cohort B (prior BCMA-directed therapy [antibody-drug conjugate or chimeric antigen receptor (CAR) T-cell therapy]). Results from cohort A after approximately 15 months of follow-up have been reported, and results from cohort B have yet to be published.14
After six 28-day cycles (24 weeks), patients who achieved a partial response or better lasting at least 2 months were eligible to switch to a dosing interval of once every 2 weeks. Of the 123 patients in cohort A, 50 patients switched to biweekly dosing, and of these patients, 40 improved or maintained their response for at least 6 months. The ORR was 61%, with 35% of patients having achieved a CR or better. MRD negativity was achieved in 89.7% of patients with a CR or better who were evaluable for MRD. The secondary end points of median duration of response, progression-free survival, and overall survival had not been reached at a median follow-up of 14.7 months.14,15
Extended follow-up approximately 15 months after the last patient’s first dose with a data cutoff date of March 14, 2023, have been reported (Table 2).
Talquetamab
The MonumenTAL-1 trial assessed the efficacy and safety of talquetamab with 2 dosing cohorts: weekly and every other week.3 Patients either received talquetamab at 405 μg/kg weekly or at 800 μg/kg every other week. The majority of patients were triple-class refractory across the weekly dosing group (n = 143) and every-other-week dosing group (n = 145) and among those who had received prior T-cell receptor therapy (n = 51). At the median follow-up of 11.7 months for the group receiving the 405-μg/kg dose and 4.2 months for the group receiving the 800-μg/kg dose, the ORR was 70% and 64%, respectively. For the group receiving the 405-μg/kg dose, the median time to response and to a CR were 0.9 (range, 0.2-3.8) and 9.3 months (range, 1.7-17.1), respectively, and for the group receiving the 800-μg/kg dose, these were 1.2 (range, 0.3-6.8) and 2.3 months (range, 2.1-6.8), respectively. The median duration of response for those receiving the 405 μg/kg and 800-μg/ kg doses were 10.2 (95% CI, 3.0-NR) and 7.8 months (95% CI, 4.6-NR), respectively.16
Extended follow-up with a data cutoff date of January 23, 2023, have been reported (Table 2).
Practical Considerations
CRS and neurologic toxicity
CRS and neurologic toxicity, including ICANS, are well-recognized complications associated with immune effector cell therapies such as bispecific TCEs. However, the incidence, severity, and onset of CRS and neurologic toxicity for TCEs vs available CAR T-cell therapies differ— specifically because TCEs are drugs with defined half-lives dosed intermittently.6,16 CRS and ICANS, which may appear as fever, malaise, hypotension, respiratory distress, and neurological manifestations (Table 2), require prompt identification, evaluation, and management.17 Furthermore, it is critical to consider a differential diagnosis in patients experiencing CRS or ICANS, given the possibility of an underlying infection or other medical condition that may mimic CRS or ICANS. Infection-related symptoms, such as fever, malaise, confusion, or respiratory distress, may overlap with those observed in CRS and ICANS. Thus, a workup for infection, which may include blood and urine cultures and chest radiography, should be conducted if fever is present. Educating patients and nurses with respect to the signs and symptoms of CRS and ICANS is crucial for prompt and effective care. Furthermore, in the case of anticytokine therapy with tocilizumab (Actemra; Genentech), communication within the medical team is necessary to minimize the time between when the medication order is placed and drug administration.
CRS and ICANS are systemic inflammatory responses mediated by the overactivation of a patient’s immune system after treatment with immune effector cell therapy caused by activation of T lymphocytes, monocytes, and macrophages, leading to the downstream production of interferon-γ, IL-6, and IL-10.18 This immune activation is observed during the step-up dose phase or with the first treatment dose of TCEs. In clinical practice, the effective management of CRS and ICANS requires timely intervention and selection of appropriate therapeutic agents based on the severity of the syndrome.6 Health care centers utilize preventive measures to mitigate CRS and ICANS, including premedications, step-up doses, and inpatient monitoring. Pharmacologic agents come into play if patients experience signs of CRS or ICANS, including antipyretics, antiepileptic drugs, corticosteroids, tocilizumab, and anakinra (Kineret; Swedish Orphan Biovitrum AB).17
Monitoring, risk stratification, and prophylaxis for CRS and ICANS
Recommendations on hospitalization to monitor CRS and ICANS are present in the package insert of available TCEs.10-12 The duration of hospitalization may vary for each TCE based on how institutions implement the recommended minimum timing between step-up doses and the first treatment dose. Because institutional resource constraints and site-of-care cost considerations may limit the wider availability and use of these agents, strategies to address these constraints are emerging, such as outpatient monitoring and administration of TCEs, use of prophylactic agents for CRS, and risk stratification for CRS.
Outpatient CAR T-cell infusion and monitoring have been described by multiple centers, and experiences on outpatient step-up dosing with TCEs for patients with MM are growing, highlighting the resources needed to educate patients and the medical team regarding the necessary procedures for patient monitoring, symptom triage, and provider assessment.19-22 Of note, implementation of TCEs at institutions that do not routinely administer CAR T-cell infusions may be a challenge because resources may vary drastically compared with authorized CAR T-cell treatment centers.
Given the diversity of patient needs across institutions, the implementation of policies and procedures for monitoring patients receiving TCEs will vary. Efforts from care staff performing patient follow-ups and assisting in patient triage may be accompanied by tools such as remote vitals monitoring and rationale for TCE administration times that take into account the median time to onset of CRS. Early engagement of the multidisciplinary care team is key. Furthermore, prophylactic strategies, such as with tocilizumab, may attenuate the incidence and severity of CRS, which may further increase the feasibility of outpatient step-up dosing and monitoring.23,24 Although the use of tocilizumab for CRS prophylaxis is promising, tailored use may increase the feasibility of this practice for institutions. Notably, there is currently a paucity of data on the utility of continued dexamethasone administration beyond its use as a premedication for CRS prophylaxis.
Models for CRS risk stratification may aid clinical decision-making for more intensive monitoring or prophylactic measures for patients at higher risk of CRS, particularly grade 2 or higher. However, data on risk factors of CRS with TCEs are still insufficient to build such models at this time.
Management of CRS
CRS may be graded in terms of severity based on the presence of fever, hypotension, and/or hypoxia. For the management of low-grade CRS, an antipyretic and intravenous hydration act as primary interventions. The antipyretic is administered to alleviate mild symptoms before considering more specific interventions, such as tocilizumab or other pharmacologic agents for moderate to severe cases of CRS. Tocilizumab, an IL-6 receptor inhibitor, can also be considered if fever persists with grade 1 CRS despite adequate supportive care. IL-6 is notable as a cytokine commonly found at elevated levels in individuals experiencing CRS, with its increased concentrations often associated with the severity of the syndrome.6 Tocilizumab can be repeated every 8 hours if there is no improvement of CRS signs and symptoms but is limited to 3 doses within 24 hours and a maximum of 4 total doses.25 Furthermore, it has been demonstrated that IL-6 can be blocked without affecting the therapeutic activity of T cells mediated by CD3 bispecific treatment, also suggesting benefit with earlier use of cytokine blockade, possibly prophylactically, to mitigate CRS.18
A recent analysis provided a detailed overview of CRS events observed in patients from the MajesTEC-1 trial. Of the 165 total patients, tocilizumab was used in 60 patients (36.4%) and steroids in 14 patients (8.5%). There were 195 CRS events reported (in 119 patients), and 68 of the events were managed with tocilizumab and 127 were not. Fewer events managed by tocilizumab were followed by a subsequent event vs events for which tocilizumab was not used (19.1% vs 49.6%, respectively). Forty-five patients received tocilizumab for their first CRS event, of whom 9 (20%) had a subsequent CRS event compared with 46 of 74 patients (62.2%) who were not managed with tocilizumab for their first CRS event and had a subsequent event.26
Management of ICANS
Available TCEs are associated with a lower incidence and severity of ICANS compared with available CAR T-cell therapies. However, prompt evaluation, diagnosis, and management of ICANS remain vital in the care of patients receiving these therapies. ICANS is graded using the Immune Effector Cell–Associated Encephalopathy (ICE) score.16 The ICE score comprises several clinical and neurological parameters, including orientation, naming, following commands, writing, and attention. Nonsedating antiepileptic drugs can be used for seizure management as indicated. In contrast to supportive care with CAR T-cell therapy, antiepileptic drugs are not added as standard prophylaxis with TCEs.26
Corticosteroids are a cornerstone in the treatment of ICANS, dexamethasone specifically because it has a greater central nervous system penetration compared with other corticosteroids.17 For example, dexamethasone doses may range from 10 mg daily for grade 1 ICANS to 10 mg every 6 hours for grade 2/3 ICANS. High-dose methylprednisolone (1 mg/kg) can be considered for severe ICANS. There is discordance on the use of tocilizumab for treatment of ICANS without signs or symptoms of CRS due to the risk that tocilizumab may exacerbate ICANS.26 Tocilizumab is a monoclonal antibody that cannot cross the blood-brain barrier, thus blocking the IL-6 receptor in peripheral tissues.26 Hypothetically, this can lead to a temporary increase in levels of IL-6, thereby increasing the risk of ICANS.27 Alternatively, anakinra, an IL-1 receptor antagonist that can reach clinically relevant concentrations in the central nervous system, may be a preferred adjunct to dexamethasone.28-30
The Risk Evaluation and Mitigation Strategy program
Due to the serious safety concerns arising from the risk of CRS and ICANS, TCEs with approved indication for the treatment of MM are available only through the restricted Risk Evaluation and Mitigation Strategy (REMS) drug safety program.10-12 There are important REMS considerations for MM bispecific antibodies for each of the key participants, specifically prescribers, pharmacy/health care settings, and wholesalers. Notably, there are no patient-specific requirements for a REMS program. Teclistamab and talquetamab are available under the same REMS program, in contrast to elranatamab. Prescribers must be certified by the program by enrolling and completing training, which is a 1-time requirement for each of the REMS programs. Certified prescribers must counsel patients on the risk of CRS/ICANS at therapy initiation and dispense a patient wallet card, which patients are advised to always carry with them to signify their current treatment with these bispecific antibodies. Pharmacy/health care settings must be enrolled, have a designated authorized representative for each site, and train relevant staff on REMS requirements. For each dose dispensed, a REMS dispense authorization code must be generated in the portal for the respective bispecific antibody, verifying the ordering provider’s REMS certification status. Wholesalers may only supply drug to REMS-certified pharmacy/health care settings, which may not loan, borrow, or sell these agents to other sites.10-12
Monitoring for and prophylaxis against infections
Patients with MM have an increased risk of infection due to immunodeficiencies that can be disease and treatment related.31,32 With anti-BCMA TCEs, the risk and severity of infections have been more notable and persistent than with other treatments in the MM armamentarium, which necessitate considerations for infection prevention to decrease infection-related morbidity and mortality.7,8 Notably, the use of anti-BCMA TCEs is associated with significant incidences of PJP and CMV reactivation and infection. Expert recommendations on prevention of infections for patients with MM receiving TCEs address the need for immediate guidance, acknowledging that recommendations may be updated to reflect new and maturing data from TCE therapies targeting BCMA as well as other antigens (eg, GPRC5D, Fc receptor-like 5 [FcRH5]).33,34 This is because data are more mature for trials evaluating anti-BCMA TCEs compared with TCEs against non-BCMA antigens. Current data suggest that the incidence and severity of infection may be lower with non– anti-BCMA TCEs compared with anti-BCMA TCEs.6,33,34 However, given the incidence of serious and fatal infections as well as opportunistic infections with anti-BCMA TCEs, recommendations for the prevention and management of infection for patients with MM receiving treatment with TCEs may be broadly considered until more data are available, at which time more detailed recommendations may be provided reflecting disease stage, TCE antigen, and TCEs used in combination therapy.6,33,34
Accompanying these current recommendations is interest in modifying the dose density or intensity—based on available evidence—to decrease the risk for infection that accompanies TCE therapies, such as by rationally increasing the dosing interval for patients with sustained disease response. For example, in the phase 2 portion of the MajesTEC-1 trial, patients who received teclistamab and achieved a CR or better for 6 months or more could switch from weekly dosing of 1.5 mg/kg subcutaneously to every 2 weeks; there was an additional option for a schedule of every 4 weeks if patients demonstrated sustained response to the every-2-weeks schedule.5 Patients who switched to every 2 weeks had a decreased incidence of new-onset grade 3 or higher infections and, importantly, maintained their depth of response.13 For teclistamab, the option of a decreased dosing frequency of 1.5 mg/kg subcutaneously every 2 weeks for patients who have achieved a CR or better for a minimum of 6 months has been approved by the European Commission and recently by the FDA.10 In addition, a schedule of every 2 weeks reflecting the doses used in the MagnetisMM-3 trial is FDA approved for elranatamab for patients who are responders (partial response or better) at week 25 and onward and who have maintained this response for at least 2 months.4,12
Also noteworthy are a meta-analysis (median follow-up, 7.6 months; range, 1.7-14.9) and pooled analysis (median follow-up, 6.1 months; range, 1.7-14.1) that have described the prevalence, character, and severity of infections in patients with MM treated with TCEs.7,8
In the meta-analysis that included 16 clinical trials (1666 patients), with 12 trials evaluating bispecific and trispecific TCEs as monotherapy (1477 patients) and 4 trials evaluating TCEs in a combination therapy, the prevalence of all-grade and grade 3 or greater infections was 56% and 24%, respectively; of the reported infection events, 68% were microbiologically confirmed, which included viral (49%), bacterial (45%), and fungal (6%). Sixty-five fatal infections were reported. Furthermore, the median time to onset of infections was described for all-grade infections (49-79 days) and severe infections (≥ 3 months). Two late (≥ 12 months) fatal infections were reported in 1 study.8
In the pooled analysis that included 11 clinical trials (1185 patients) evaluating a TCE as monotherapy, with 71.6% of patients with MM receiving an anti-BCMA TCE, the prevalence of all-grade and grade 3 or greater infections was 50% and 24.5%, respectively, with the risk of grade 3/4 infections higher with anti-BCMA TCEs vs non– anti-BCMA TCEs (30% vs 11.9%, respectively). Opportunistic infections were also reported, including CMV reactivation/infection, PJP, Candida esophagitis, ophthalmic herpes simplex virus (HSV), and progressive multifocal leukoencephalopathy. The prevalence of PJP and CMV reactivation and/or infection was 4.2% and 8%, respectively. Twenty-eight deaths attributed to infection were reported.7
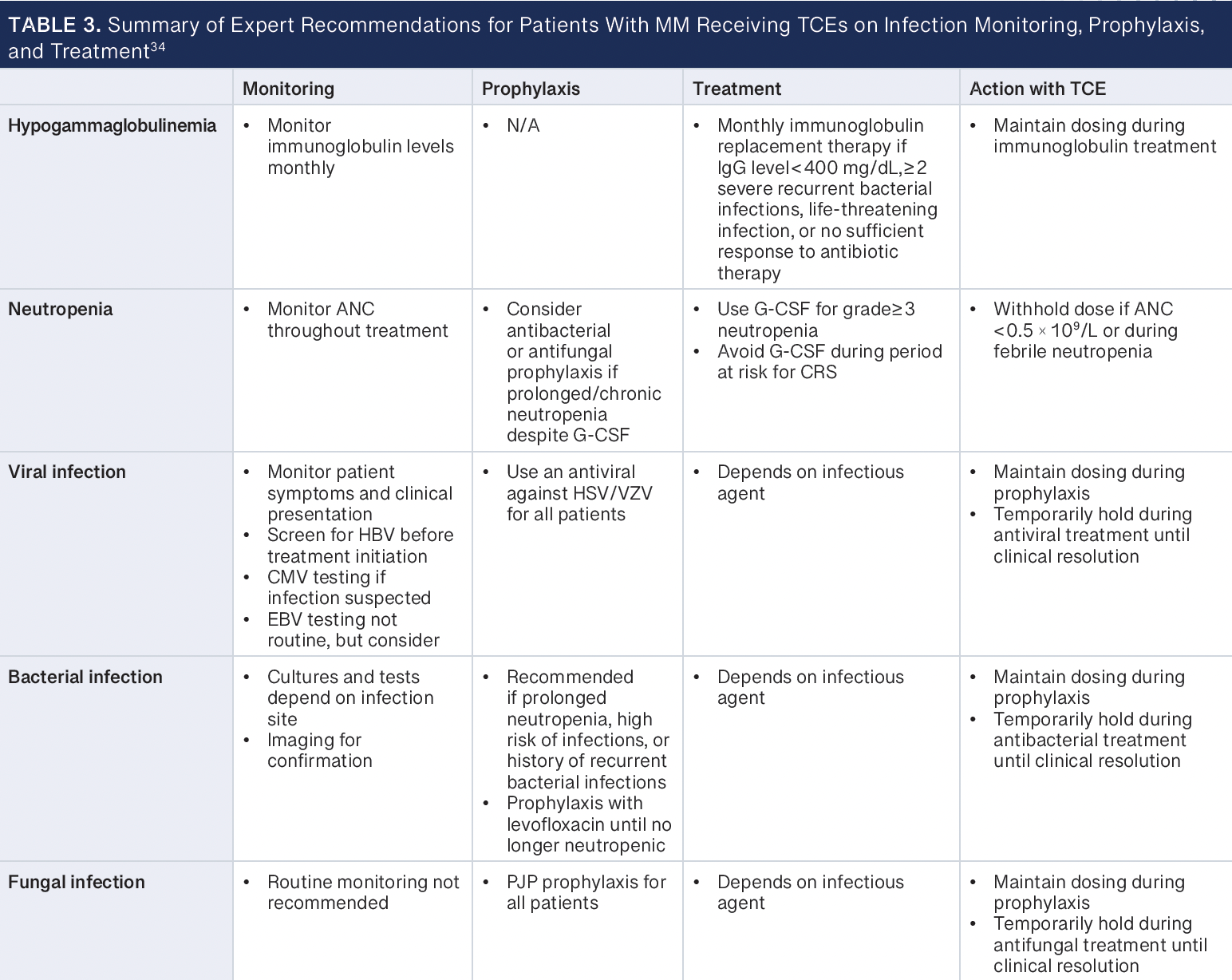
To date, several sets of expert recommendations address considerations for hypogammaglobulinemia, infection (viral, bacterial, and fungal) monitoring and prophylaxis, and vaccinations for patients with MM who are receiving therapy with TCEs, including recommendations from a panel of experienced investigators from the academic Consortium to Overcome Multiple Myeloma through Innovative Trials, from a global expert panel, and from the European Myeloma Network.6,33,34 Key considerations from these expert recommendations are summarized below and described in Table 3.34
Immunoglobulin replacement should be considered for patients who have severe hypogammaglobulinemia (IgG < 400 mg/dL), who have had 2 or more severe recurrent infections by encapsulated bacteria regardless of IgG level, who have had a life-threatening infection, or who have a documented bacterial infection with no or insufficient response to antibiotic therapy. Patients should be evaluated for monthly immunoglobulin replacement during the period of immunoparesis, in the absence of serious infections, until IgG levels are consistently more than 400 mg/dL. Due to the possible confusion between an infusion- or injection-related reaction with supplemental immunoglobulin and CRS, immunoglobulin supplementation should be deferred until after the risk of CRS with the TCE has subsided. The recommended dosage of current FDA-approved TCEs is given until disease progression or unacceptable toxicity, which may limit immunoparesis recovery, so the optimal duration of immunoglobulin replacement remains unclear; finite treatment duration with TCEs in the treatment of MM may provide additional insight (NCT05932680). Furthermore, recent experiences have continued to highlight the risk for severe hypogammaglobulinemia with BCMA-directed TCEs and CAR T cells and the association of immunoglobulin supplementation with a decreased risk of severe infection.35-37
Recommendations for monitoring for and prophylaxis against viral infections, including HSV/varicella-zoster virus (VZV), CMV, Epstein-Barr virus, and hepatitis B virus (HBV), should be considered. Prophylaxis against HSV/VZV with acyclovir or valacyclovir is recommended for patients with R/R MM on active therapy. CMV serologies may be considered prior to initiating TCE therapy, with monitoring of CMV DNA copies considered serially (eg, every 3 months) or in case of suspected infection. Data are limited on the optimal frequency for serial monitoring of CMV DNA copies, with practice varying between institutions. HBV screening is recommended and HBV reactivation risk assessment is conducted for patients with chronic or past HBV infection in consideration for prophylaxis with entecavir, tenofovir, or lamivudine, whereas patients with active HBV infection should be managed according to guidelines.38,39 Monitoring for reactivation of Epstein-Barr virus is not routinely recommended but may be considered in cases of persistent fever and fatigue.40,41
Prophylaxis with an antibacterial agent, such as levofloxacin, may be considered for patients during periods of prolonged neutropenia or for those with a history of recurrent infections and a high risk for infection. Prophylaxis may be continued until neutrophil recovery or when infectious risk has decreased. General antibacterial prophylaxis is not recommended due to the risk for promoting antimicrobial resistance. Similarly, routine prophylaxis for fungal infection, except for PJP, is not recommended, although it may be considered in consultation with an infectious disease specialist for patients during periods of prolonged neutropenia, during prolonged use of high-dose corticosteroids, or with a history of fungal infections. Prophylaxis for PJP with either sulfamethoxazole-trimethoprim, dapsone, atovaquone, or inhaled or intravenous pentamidine is recommended for all patients receiving treatment with a TCE, and the applicable prophylactic agent should be guided by patient-specific characteristics (eg, allergy to sulfonamides, which precludes use of sulfamethoxazole-trimethoprim; glucose-6-phosphate dehydrogenase deficiency, which precludes use of dapsone) and institutional guidelines.6,33,34
Granulocyte colony-stimulating factor (G-CSF) is recommended for patients with MM with severe neutropenia to decrease the risk for neutropenia-related infections. However, during periods of risk for CRS, such as during step-up dosing and the first treatment dose with TCEs, the use of G-CSF may be weighed with the potential for inducing cytokine production and exacerbating CRS.40,41 Patients should be vaccinated according to recommendations, with emphasis on vaccinations for influenza, Streptococcus pneumoniae, herpes zoster, SARS-CoV-2, and respiratory syncytial virus.42
Unique toxicities mediated by GPRC5D on keratinized tissues
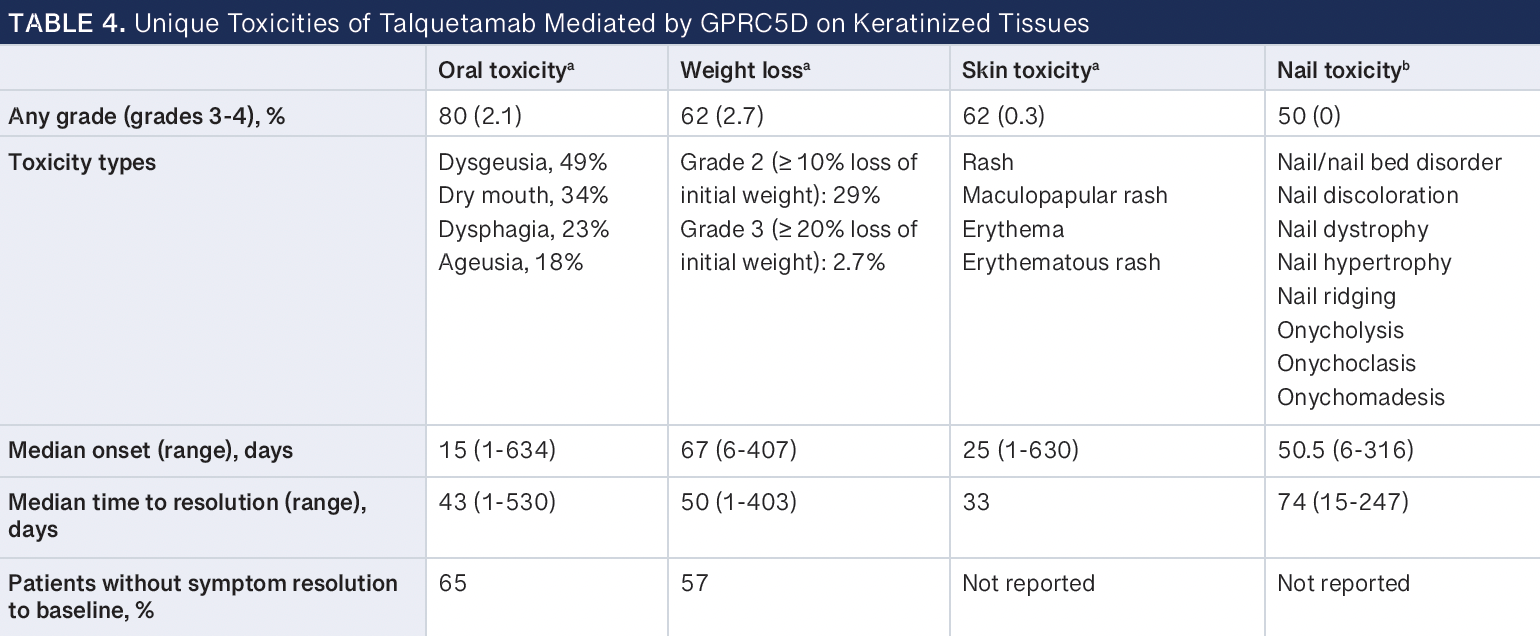
Talquetamab has a unique set of on-target, off-tumor toxicities due to GPRC5D protein expression in healthy keratinized tissues, such as cells in the oral cavity of the tongue, salivary glands, tonsils, skin, and nails.43 In clinical trials, these toxicities were commonly observed in more than 50% of patients receiving talquetamab (Table 4).3,11 Specifically, any grade of oral toxicity, weight loss, skin toxicity, and nail toxicity occurred at 80%, 62%, 62%, and 50%, respectively. Oral toxicities consisted of dysgeusia (49%), dry mouth (34%), dysphagia (23%), and ageusia (18%). Some patients also experienced significant weight loss of more than 10% of their initial weight (grade 2; 29%) and, in some cases, more than 20% of their initial weight (grade 2; 2.7%). Skin toxicities included rash, maculopapular rash, erythema, and erythematous rash. Nail toxicities were nail and nail bed disorder, nail discoloration, dystrophy, hypertrophy, ridging, onycholysis, onychoclasis, and onychomadesis. Although these were common, they were mostly low grade. However, these changes were permanent in some patients, with many reporting no symptom resolution to baseline (oral toxicity, 65%; weight loss, 57%).11
Current management strategies of on-target, off-tumor cell effects of targeting GPRC5D focus on oral toxicity and weight loss, skin toxicity, and nail toxicity.44-48 Although mostly low grade, these unusual toxicities may significantly impact patient experience and quality of life. Oral toxicities can affect a patient’s ability to experience food taste and texture, resulting in changes in diet or interest in food and further leading to weight loss and deterioration of overall nutritional status. Real-world studies report skin and nail toxicities as relatively benign, painless, and self-limiting, with some patients reporting concerns with changes in nail appearance and nail loss.44,49 GPRC5D toxicity management for taste alterations include food texture and flavor experimentation and adequate hydration. Salivary substitutes, such as salt mouth rinse, artificial saliva spray, and corticosteroid mouth wash (eg, dexamethasone oral solution), for dry mouth have been reported to good effect.44 Ongoing vitamin and nutritional support may be necessary throughout treatment with talquetamab, with dose modification and dose withholding as necessary. Supportive measures for skin and nail toxicities include the use of heavy moisturizers and lotions and topical corticosteroids to control inflammation, irritation, and redness; oral corticosteroids may be considered for severe events.44 Patient education and early detection and intervention are key to managing these adverse events long term.
Safety considerations: Vial sizes/concentrations, stability, and administration
There are several practical considerations for safely preparing, storing, and administering teclistamab, elranatamab, and talquetamab. Highlighted in Table 5 are some key differences from each product’s prescribing information.10,12
About the Authors
Matthew M. Lei, PharmD, BCOP, is a clinical pharmacy specialist—lymphoma in the Jon and Jo Ann Hagler Center for Lymphoma at Massachusetts General Hospital in Boston.
Jack Malespini, PharmD, BCOP, is a clinical pharmacy specialist in the Department of Pharmacy at Massachusetts General Hospital in Boston.
Erica Tavares, PharmD, BCPS, BCOP, is a clinical pharmacy specialist in the Department of Pharmacy at Massachusetts General Hospital in Boston, Massachusetts.
Sarah O’Neill, PharmD, is a clinical pharmacy specialist in the Department of Pharmacy at Massachusetts General Hospital in Boston.
Diana Cirstea, MD, is an attending physician in the Center for Myeloma at Mass General Cancer Center in Boston.
E. Bridget Kim, PharmD, BCPS, BCOP, is a clinical pharmacy specialist in the Department of Pharmacy at Massachusetts General Hospital and in the Center for Myeloma at Mass General Cancer Center in Boston.
Conclusion
The emergence of TCEs, such as teclistamab, elranatamab, and talquetamab, marked a watershed moment in the therapeutic armamentarium for R/R MM, offering promise for patients navigating the available complex treatment options. In this review, we have summarized the pivotal data that led to the approval of these therapies while highlighting their efficacy and safety. Furthermore, we emphasize that the integration of these therapies into patient care requires supportive care considerations for CRS and neurotoxicity, infection, and antigen-specific toxicities. These efforts will remain important not only as available TCEs are investigated in combination with other therapies (eg, anti-CD38 monoclonal antibodies, IMiDs, cereblon E3 ligase modulators, CAR T cells, T-cell costimulatory molecules, and other TCEs) but also as TCEs targeting multiple tumor antigens (eg, trispecific TCEs) or other novel antigens (eg, FcRH5) become available.
Furthermore, although questions remain about the optimal sequencing of not only TCEs and CAR T-cell therapies but also antigen targets (BCMA, GPRC5D, and FcRH5), available data demonstrate that TCEs retain efficacy in patients previously treated with CAR T cells and suggest that patients who have progressed on or were refractory to a TCE may have a blunted response to CAR T cells.50-54 As broad efforts to implement TCEs into clinical practice continue, challenges and opportunities will remain because of ongoing investigation of TCEs in earlier lines of therapy. Considerations for agent-specific toxicity management, including CRS, neurotoxicity, and antigen-specific toxicities, are evolving alongside emerging real-world experiences. Importantly, strategies to facilitate safe outpatient step-up dosing of TCEs may allow broader availability and access to these therapies. The integration of evolving supportive care strategies remains a focus in the care of patients with MM. This becomes particularly important as we aim to incorporate T-cell–engaging therapies into earlier lines of treatment, with the goal of inducing deeper, more durable responses while pursuing the possibility of curing patients with MM.
References
Lemaire M, Deleu S, De Bruyne E, Van Valckenborgh E, Menu E, Vanderkerken K. The microenvironment and molecular biology of the multiple myeloma tumor. Adv Cancer Res. 2011;110:19-42. doi:10.1016/b978-0-12-386469-7.00002-5
Branagan A, Lei M, Lou U, Raje N. Current treatment strategies for multiple myeloma. JCO Oncol Pract. 2020;16(1):5-14. doi:10.1200/jop.19.00244
Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232-2244. doi:10.1056/NEJMoa2204591
Lesokhin AM, Tomasson MH, Arnulf B, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29(9):2259-2267. doi:10.1038/s41591-023-02528-9
Moreau P, Garfall AL, van de Donk NWCJ, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387(6):495-505. doi:10.1056/NEJMoa2203478
Ludwig H, Terpos E, van de Donk N, et al. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: a consensus report of the European Myeloma Network. Lancet Oncol. 2023;24(6):e255-e269. doi:10.1016/s1470-2045(23)00159-6
Mazahreh F, Mazahreh L, Schinke C, et al. Risk of infections associated with the use of bispecific antibodies in multiple myeloma: a pooled analysis. Blood Adv. 2023;7(13):3069-3074. doi:10.1182/bloodadvances.2022009435
Reynolds G, Cliff ERS, Mohyuddin GR, et al. Infections following bispecific antibodies in myeloma: a systematic review and meta-analysis. Blood Adv. 2023;7(19):5898-5903. doi:10.1182/bloodadvances.2023010539
Nath K, Costa BA, Mailankody S. GPRC5D as a novel immunotherapeutic target in multiple myeloma. Nat Rev Clin Oncol. 2023;20(5):281-282. doi:10.1038/s41571-023-00735-4
Tecvayli. Prescribing information. Janssen Biotech Inc; October 2022. Accessed March 14, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761291s000lbl.pdf
Talvey. Prescribing information. Janssen Biotech Inc; August 2023. Accessed March 14, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761342s000lbl.pdf
Elrexfio. Prescribing information. Pfizer Inc; August 2023. Accessed March 14, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761345s000lbl.pdf
van de Donk NWCJ, Moreau P, Garfall AL, et al. Long-term follow-up from MajesTEC-1 of teclistamab, a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2023;41(suppl 16):8011. doi:10.1200/JCO.2023.41.16_suppl.8011
Mohty M, Tomasson MH, Arnulf B, et al. S196: elranatamab, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, for patients with relapsed/refractory multiple myeloma: extended follow up and biweekly administration from MAGNETISMM-3. Hemasphere. 2023;7(suppl 3):e1309654. doi:10.1097/01.Hs9.0000967696.13096.54
Mohty M, Tomasson MH, Arnulf B, et al. Elranatamab, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, for patients (pts) with relapsed/refractory multiple myeloma (RRMM): extended follow up and biweekly administration from the MagnetisMM-3 study. J Clin Oncol. 2023;41(suppl 16):8039. doi:10.1200/JCO.2023.41.16_suppl.8039
Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. doi:10.1016/j.bbmt.2018.12.758
Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. 2021;39(35):3978-3992. doi:10.1200/jco.21.01992
Li J, Piskol R, Ybarra R, et al. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci Transl Med. 2019;11(508):eaax8861. doi:10.1126/scitranslmed.aax8861
Borogovac A, Keruakous A, Bycko M, et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: experience from a tertiary care center. Bone Marrow Transplant. 2022;57(6):1025-1027. doi:10.1038/s41409-022-01664-z
Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4):e002056. doi:10.1136/jitc-2020-002056
Nina Varshavsky-Yanovsky A, Styler M, Khanal R, Abdelmessieh P, Fung H. P940: an outpatient model for teclistamab step-up dosing administration - initial experiences at Fox Chase Cancer Center BMT Program. Hemasphere. 2023;7(suppl 3):e605007f. doi:10.1097/01.HS9.0000970664.60500.7f
Bansal R, Paludo J, Corraes AMS, et al. Outpatient practice utilization for CAR-T and T cell engager in patients with lymphoma and multiple myeloma. J Clin Oncol. 2023;41(suppl 16):1533. doi:10.1200/JCO.2023.41.16_suppl.1533
van de Donk NWCJ, Garfall AL, Benboubker L, et al. Evaluation of prophylactic tocilizumab (toci) for the reduction of cytokine release syndrome (CRS) to inform the management of patients (pts) treated with teclistamab in MajesTEC-1. J Clin Oncol. 2023;41(suppl 16):8033. doi:10.1200/JCO.2023.41.16_suppl.8033
Trudel S, Bahlis NJ, Spencer A, et al. Pretreatment with tocilizumab prior to the CD3 bispecific cevostamab in patients with relapsed/refractory multiple myeloma (RRMM) showed a marked reduction in cytokine release syndrome incidence and severity. Blood. 2022;140(suppl 1):1363-1365. doi:10.1182/blood-2022-159381
Martin TG, Mateos MV, Nooka A, et al. Detailed overview of incidence and management of cytokine release syndrome observed with teclistamab in the MajesTEC-1 study of patients with relapsed/refractory multiple myeloma. Cancer. 2023;129(13):2035-2046. doi:10.1002/cncr.34756
Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511. doi:10.1136/jitc-2020-001511
Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112(10):3959-3964. doi:10.1182/blood-2008-05-155846
Wehrli M, Gallagher K, Chen YB, et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J Immunother Cancer. 2022;10(1):e003847. doi:10.1136/jitc-2021-003847
Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123-3127. doi:10.1182/bloodadvances.2020002328
Gazeau N, Liang EC, Wu QV, et al. Anakinra for refractory cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T cell therapy. Transplant Cell Ther. 2023;29(7):430-437. doi:10.1016/j.jtct.2023.04.001
Balmaceda N, Aziz M, Chandrasekar VT, et al. Infection risks in multiple myeloma: a systematic review and meta-analysis of randomized trials from 2015 to 2019. BMC Cancer. 2021;21(1):730. doi:10.1186/s12885-021-08451-x
Encinas C, Hernandez-Rivas JÁ, Oriol A, et al; GEM/PETHEMA (Grupo Español de Mieloma/Programa para el Estudio de la Terapéutica en Hemopatías Malignas) cooperative study group. A simple score to predict early severe infections in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2022;12(4):68. doi:10.1038/s41408-022-00652-2
Mohan M, Chakraborty R, Bal S, et al. Recommendations on prevention of infections during chimeric antigen receptor T-cell and bispecific antibody therapy in multiple myeloma. Br J Haematol. 2023;203(5):736-746. doi:10.1111/bjh.18909
Raje N, Anderson K, Einsele H, et al. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: consensus recommendations from an expert panel. Blood Cancer J. 2023;13(1):116. doi:10.1038/s41408-023-00879-7
Lancman G, Parsa K, Kotlarz K, et al. IVIg use associated with ten-fold reduction of serious infections in multiple myeloma patients treated with anti-BCMA bispecific antibodies. Blood Cancer Discov. 2023;4(6):440-451. doi:10.1158/2643-3230.Bcd-23-0049
Garfall AL, Stadtmauer EA. Understanding infection risk with anti-BCMA bispecific antibodies. Blood Cancer Discov. 2023;4(6):427-429. doi:10.1158/2643-3230.Bcd-23-0157
Little JS, Tandon M, Hong JS, et al. Respiratory infections predominate after day 100 following B-cell maturation antigen-directed CAR T-cell therapy. Blood Adv. 2023;7(18):5485-5495. doi:10.1182/bloodadvances.2023010524
Hwang JP, Feld JJ, Hammond SP, et al. Hepatitis B virus screening and management for patients with cancer prior to therapy: ASCO provisional clinical opinion update. J Clin Oncol. 2020;38(31):3698-3715. doi:10.1200/jco.20.01757
Raje NS, Anaissie E, Kumar SK, et al. Consensus guidelines and recommendations for infection prevention in multiple myeloma: a report from the International Myeloma Working Group. Lancet Haematol. 2022;9(2):e143-e161. doi:10.1016/s2352-3026(21)00283-0
Miller KC, Johnson PC, Abramson JS, et al. Effect of granulocyte colony-stimulating factor on toxicities after CAR T cell therapy for lymphoma and myeloma. Blood Cancer J. 2022;12(10):146. doi:10.1038/s41408-022-00741-2
Liévin R, Di Blasi R, Morin F, et al. Effect of early granulocyte-colony-stimulating factor administration in the prevention of febrile neutropenia and impact on toxicity and efficacy of anti-CD19 CAR-T in patients with relapsed/refractory B-cell lymphoma. Bone Marrow Transplant. 2022;57(3):431-439. doi:10.1038/s41409-021-01526-0
Ludwig H, Boccadoro M, Moreau P, et al. Recommendations for vaccination in multiple myeloma: a consensus of the European Myeloma Network. Leukemia. 2021;35(1):31-44. doi:10.1038/s41375-020-01016-0
Inoue S, Nambu T, Shimomura T. The RAIG family member, GPRC5D, is associated with hard-keratinized structures. J Invest Dermatol. 2004;122(3):565-573. doi:10.1046/j.0022-202X.2004.12628.x
Catamero D, Purcell K, Ray C, et al. Practical management of patients with relapsed/refractory multiple myeloma receiving talquetamab, a GPRC5DxCD3 bispecific antibody: experience in MonumenTAL-1. Poster presented at: 20th International Myeloma Society Annual Meeting; September 27-30, 2023; Athens, Greece.
Mancia SS, Farrell A, Louw K, et al. Characterization and Management of Oral and Dermatological Toxicities in Patients Receiving the CD3 X GPRC5D Bispecific Antibody Talquetamab (JNJ-64407564) for the Treatment of Relapsed and/or Refractory Multiple Myeloma. Blood. 2021;138(suppl_1):1658. doi:10.1182/blood-2021-153817
Jané-Salas E, Escobar-Álvarez Y, Álvarez-García R, et al. Multidisciplinary consensus on oral care in cancer patients. 2021. Accessed March 26, 2024. https://seom.org/images/Multidisciplinary_consensus_on_oral_care_cancer_patients.pdf
Mailankody S, Devlin SM, Landa J, et al. GPRC5D-Targeted CAR T Cells for Myeloma. N Engl J Med. 2022;387(13):1196-1206. doi:10.1056/NEJMoa2209900
Mittal S, Khunger N, Kataria SP. Nail Changes With Chemotherapeutic Agents and Targeted Therapies. Indian Dermatol Online J. 2022;13(1):13-22. doi:10.4103/idoj.IDOJ_801_20
Purcell K, CatameroD, DaiV, et al. Management considerations for dermatologic toxicities associated with talquetamab, a GPRC5D×CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. Presented at: 20th International Myeloma Society Annual Meeting: Nursing Symposium; September 27-30, 2023; Athens, Greece.
Cohen AD, Mateos MV, Cohen YC, et al. Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood. 2023;141(3):219-230. doi:10.1182/blood.2022015526
Touzeau C, Krishnan AY, Moreau P, et al. Efficacy and safety of teclistamab (tec), a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in patients (pts) with relapsed/refractory multiple myeloma (RRMM) after exposure to other BCMA-targeted agents. J Clin Oncol. 2022;40(suppl 16):8013. doi:10.1200/JCO.2022.40.16_suppl.8013
Touzeau C, Schinke C, Minnema M, et al. S191: Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DxCD3 bispecific antibody (BsAb), for relapsed/refractory multiple myeloma (RRMM).Hemasphere. 2023;7(Suppl ):e5955094. doi:10.1097/01.HS9.0000967676.59550.94
Chari A, Touzeau C, Schinke C, et al. Talquetamab, a G Protein-Coupled Receptor Family C Group 5 Member D x CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM): Phase 1/2 Results from MonumenTAL-1. Blood. 2022;140(suppl_1):384-387. doi:10.1182/blood-2022-159707
Manier S, Lesokhin A, Mohty M, et al. P870: efficacy and safety of elranatamab in patients with relapsed/refractory multiple myeloma and prior B-cell maturation antigen (BCMA)-directed therapies: a pooled analaysis from MagnetisMM studies. Hemasphere. 2023;7(suppl 3):e26808c7. doi:10.1097/01.HS9.0000970384.26808.c7
Disclosures
The authors have no disclosures.