About the Authors
Laura Gillespie, PharmD, is an antimicrobial stewardship pharmacist and pharmacy resident preceptor at Saint Joseph Health System in Mishawaka, Indiana.
Yassmeen Marzuq, PharmD, is a preceptor and clinical pharmacist at Saint Joseph Health System in Mishawaka, Indiana.
Anthony V. Thomas, BS, is a student at Indiana University School of Medicine–South Bend in South Bend, Indiana.
Connor M. Bunch, MD, is an EM/IM-2 resident at Henry Ford Hospital in Detroit, Michigan.
Abstract
Purpose
Clostridioides difficile contributes to rising rates of health care–associated infections (HAIs). Hospital antimicrobial stewardship programs and pharmacist-led initiatives may aid in reducing cases of both health care facility–onset (HO) and community-onset (CO) health care facility–associated (HCFA) C difficile infections (CDIs).
Methods
Retrospective data were collected from 2018 through 2021 for CDI prevalence, associated mortality, and standardized infection ratio (SIR). Statistical analyses for C difficile SIR data used a negative binomial regression model to calculate expected vs predicted number of infections.
Interventions
Pharmacist-led initiatives included (1) an antibiotic guidelines book, (2) β-lactam allergy education, (3) implementation of a penicillin skin testing protocol, and (4) improved fluoroquinolone prescribing appropriateness.
Results
HO-CDIs decreased from 35 cases in 2018 to 7 cases in 2021 (80% reduction), and CO-HCFACDIs decreased from 37 cases in 2018 to 7 in 2021 (81% reduction). Total fluoroquinolone reduction from 2018 to 2021 was 74% (19 vs 74 days of therapy per 1000 days at risk). The HOCDI SIR decreased from a yearly average rate of 0.74 in 2018 to 0.25 in 2021 (66% decrease). Furthermore, all-cause mortality in patients with HO-CDI decreased from 6 deaths in 2018 to 2 deaths in 2021.
Conclusion
Utilization of pharmacist-led initiatives may play a significant role in helping to reduce CDI rates and associated mortality and health care costs.
Introduction
Clostridioides difficile is the most common pathogen to cause health care–associated infections (HAIs) within the United States.1,2 Per a 2019 CDC report, C difficile resulted in at least 12,800 American deaths in 2017.3 Additionally, treatment of C difficile causes significant health care burden, costing the United States $4.8 billion each year in excess costs.4
The prevalence of C difficile infections (CDIs) is largely due to the overuse of unnecessary and broad-spectrum antimicrobials and has become an area of focus for most antimicrobial stewardship (AMS) programs.2,3 Although there are many studies showing that AMS efforts decrease CDI rates, limited papers outline multifaceted and sustainable interventions that significantly decrease the rates of CDIs within a health care facility.5-11
The purpose of this study is to provide a description of several antimicrobial stewardship initiatives that were implemented in a community hospital setting and to report the associated changes in rates of health care facility–onset (HO) CDIs and community-onset (CO) health care facility–associated (HCFA) CDIs.
Methods
From January 2018 to October 2021, the hospital’s AMS pharmacist focused on interventions that increased appropriate antimicrobial use. CDI rates were collected through the BD MedMined platform, which directly links to electronic health records (EHRs) and reports positive cases to the National Healthcare Safety Network (NHSN) database. Each positive C difficile result was further delineated into the categories of HO-HCFA and COHCFA by the hospital’s infection prevention department. Although the AMS team kept track of the total raw number of CDIs, the trending of rates was more accurately assessed utilizing a standardized infection ratio (SIR), a statistic used to track HAIs over time that compares the actual number vs the predicted number of infections (ratio of observed infections [O] to expected infections [E; O/E]). The predicted number of infections is a value given by the NHSN as an estimate based on national baseline data and is risk adjusted for hospitals with higher-acuity patients. The SIR also adjusts for health care systems’ census or total patient days at risk (DAR).
The BD MedMined platform was also utilized to acquire data on the use of fluoroquinolones (includes the 2 formulary agents, ciprofloxacin [Cipro; Pfizer] and levofloxacin [Levaquin; Sanofi-Aventis], and oral vancomycin [Firvanq; Azurity Pharmaceuticals] and fidaxomicin [Dificid; Merck]). Antimicrobial usage was sent directly to the hospital on a quarterly basis by BD MedMined and reported as days of therapy (DOT) per 1000 DAR (DOT/1000 DAR). Ciprofloxacin and levofloxacin data were assessed to help analyze an association between decreased fluoroquinolone usage and CDI rates. Oral vancomycin and fidaxomicin data were collected as a second method of indirectly measuring hospital CDI rates.
Statistical analyses for the HO-CDI SIR data were provided by the NHSN utilizing a negative binomial regression model to calculate the number of predicted infections under the 2015 national HAI aggregate baseline data. The reported P values were calculated using a mid-P exact test and indicated whether the number of observed infections was significantly different from the predicted number.
The Pearson correlation coefficient was utilized to determine the correlation between both CDI rates (cases/10,000 DAR) and oral vancomycin and fidaxomicin use (DOT/1000 DAR) as well as the correlation between CDI rates (cases/10,000 DAR) and fluoroquinolone use (DOT/1000 DAR).
Research for this study was deemed exempt from institutional review board review and approval.
Interventions
Several initiatives were implemented by the AMS team in an effort to improve antimicrobial utilization and their associated sequelae: a local health care system–specific antibiotic guidelines book, β-lactam allergy education, initiation of penicillin allergy skin testing, and decreased utilization of fluoroquinolones.
Antibiotic Guidelines Book. In 2018, fluoroquinolone and other broad-spectrum antibiotic utilization rates were reported at far above national averages through the BD MedMined Surveillance Advisor database. In an effort to improve empiric indication-specific antibiotic selection, the AMS pharmacist and infectious disease (ID) physician published a hospital-specific antibiotic guidelines book in early 2019. This book combined national guidelines with local antibiogram data, dose optimization strategies, guidance on β-lactam allergies, and a chart of the Clinical and Laboratory Standards Institute breakpoints, highlighting breakpoints for the hospital’s most encountered organisms vs antimicrobials. Flowcharts were created for the most overtreated indications to help guide assessment, lab test interpretations, and utilization of the most narrow-spectrum coverage.
β-Lactam Allergy Education. The hospital’s AMS pharmacist identified the hospital overutilized alternate, non-penicillin, non-cephalosporin antibiotics, such as fluoroquinolones, clindamycin (Cleocin T; Pfizer), and carbapenems, for the treatment and prophylaxis of infections due to patient-stated penicillin allergies. Approximately 10% to 20% of the United States population reports an allergy to penicillin antibiotics; however, only 10% of self-reports of penicillin allergy are accurate, putting the rate of a true allergy at less than 1% of patients.12-15 These implications pose a significant barrier to AMS, with important clinical and economic implications, including increased antimicrobial resistance and potential development of CDIs. For example, it has been previously demonstrated that patients with a penicillin allergy label have a 23% higher incidence of CDIs compared with individuals who do not have a listed penicillin allergy.15
The concerns noted above prompted creation of a document in 2018 titled “Beta-Lactam Cross-Reaction Tidbits,” which included information on what most likely causes a cross-reaction, which occurs through the sharing of chemical structural side chains.16-19 This document included a chart of the penicillins and cephalosporins that share similar clinically relevant side chains as well as cephalosporins that share similarly clinically relevant side chains with other cephalosporins. This document highlighted that not only can a patient who has a true penicillin allergy receive most cephalosporins and vice versa, but also an individual may utilize certain cephalosporins while having a true documented allergy to another cephalosporin.16,17
The AMS pharmacist provided education pertaining to the information included in this document to clinicians in informal, department-specific talks as well as formal continuing medical education and lectures. This information was also brought to the Indiana Hospital Association’s antimicrobial stewardship work group in a collaboration to publish state-specific information in a joint effort with the Sanford Guide’s mobile application and online resource, Sanford Guide Web Edition.
Penicillin Skin Testing. Per literature discussions on β-lactam allergies, one way to optimize antimicrobial therapy, reduce adverse events and drug acquisition costs, and minimize development of antibiotic resistance and emergence of pathogenic organisms is to implement a penicillin skin testing (PST) program.20-22 The previous work of providing comprehensive β-lactam allergy education paved the way for the AMS pharmacist to begin a PST program for qualifying hospitalized patients at the end of 2018, with testing commencing in 2019. The program involved collaboration with the ordering physician for a PST, a thorough allergy history assessment directly with the patient, and performance of a skin test, if appropriate. Although it was found that the PST program itself in small numbers may not have a large CDI rate impact, collaboration with providers and other health care staff for each allergy assessment does. The AMS pharmacist also recognized the profound effect of spending time talking with patients about stated allergies.
Fluoroquinolone Utilization. Due to the hospital’s high utilization of fluoroquinolones, along with literature stating this class of antibiotics’ association with the most toxigenic strain of C difficile, the AMS pharmacist began an aggressive approach in mid–second quarter 2019 to reduce use of fluoroquinolones.23,24 Initially, a 1-month fluoroquinolone medication utilization evaluation (MUE) was performed to better assess fluoroquinolone use and delineate inappropriate prescribing. Results of the MUE were reviewed with prescribers and criteria for “appropriate” vs “inappropriate” prescribing were discussed, along with education regarding the warnings associated with this drug class. Use of a fluoroquinolone was considered “appropriate” if (1) there were no other more appropriate alternate options (such as a β-lactam), taking into consideration the disease state(s) and/or identified organism with susceptibilities, and confirmed allergy status; and (2) it was utilized as transition to oral therapy, when oral β-lactams or other alternates were not appropriate (eg, completion of therapy for Pseudomonas aeruginosa infection or gram-negative rod bacteremia). All other cases not meeting “appropriate” criteria were considered “inappropriate.” The MUE was created and reviewed by the AMS pharmacist in close collaboration with the ID physician if a case was considered a gray area for appropriateness. The AMS pharmacist provided real-time, comprehensive allergy assessments and/or penicillin allergy testing for each of the determined “inappropriate” allergy patients. Additionally, the AMS pharmacist provided education on appropriate fluoroquinolone use to each of the prescribers at that time.
Following the initial fluoroquinolone 1-month MUE, the AMS pharmacist continued to run a report on fluoroquinolone use on most days during the week. Restriction of fluoroquinolones had been discussed between the AMS pharmacist and the ID physician; however, the AMS team felt this may lead to an excessive overutilization of ID consults and possible delays in antimicrobial therapy. Furthermore, total utilization had decreased significantly after this initial MUE and continued ongoing review with providers, and left the pharmacist with a manageable list of patients to assess as part of daily patient AMS rounding.
Additionally, the AMS pharmacist educated other pharmacists about use of fluoroquinolones and shared the results of the MUE. Clinical pharmacists for each care team evaluated the need for fluoroquinolones upon verification and through chart review to further limit their use.
Modifications to the computer’s order entry system to aid in the selection of appropriate antibiotic use was discussed during this process but could not be implemented at that time due to an impending change to another EHR platform at the end of 2021. Once the new system went live, the ordering of antibiotics required input of a specific indication and anticipated duration of therapy. This further helped with pharmacist interventions in real time and therapies could be discontinued or duration of therapy could be modified.
Results
PST Rates. On average, approximately 12 PST orders were placed per month throughout 2019 and into early 2020. Testing rates significantly dropped in the latter part of 2020 and into 2021 due to COVID-19. Although skin testing did slow in the second half of the study period, the momentum of work with allergy assessments, patient discussions, and analyzation of cross-reactivities remained constant.
HO- and CO-HCFA-CDI Rates. Prior to implementation of the stated pharmacist-led initiatives, total yearly HO- and CO-HCFA-CDI cases from the study years 2018 through 2020 were 35 and 37 in 2018; 17 and 19 in 2019; 11 and 7 in 2020; and 7 and 7 in 2021, respectively. Following implementation of pharmacist-led initiatives, the total decrease in HO-CDI rates from 2018 to 2021 was 80%; the decrease in CO-HCFA-CDI rates from 2018 to 2021 was 81%.
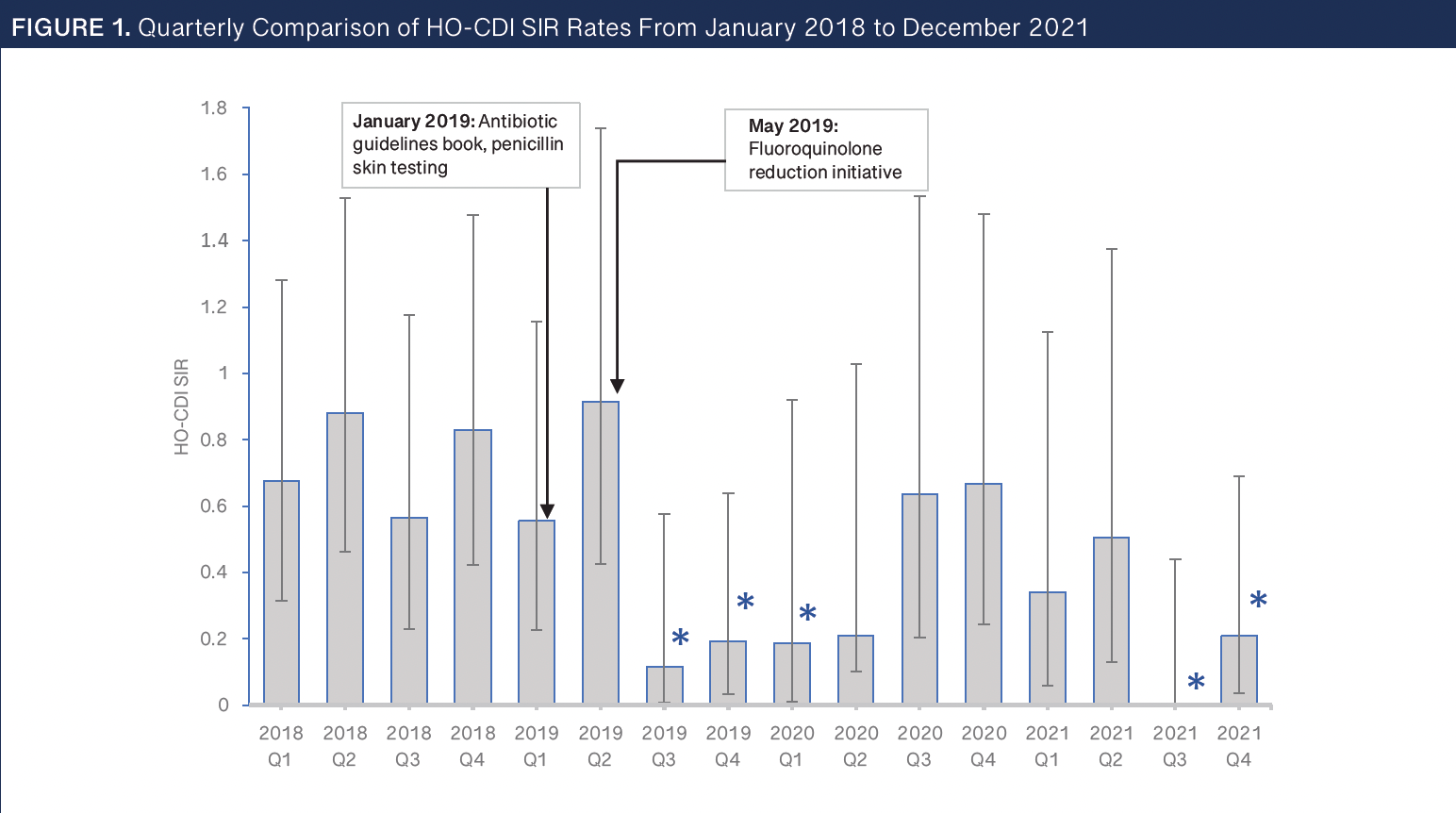
HO-CDI SIR Rate Trend. To more accurately interpret infection rates over time, CDI rates were assessed utilizing an SIR. Figure 1 shows the trend of the HO-CDI quarterly SIR data from 2018 through 2021 and includes an asterisk over the quarters in which NHSN calculated a significant difference of observed vs predicted infections. Overall, yearly average SIR rates decreased from 0.74 in 2018 to 0.25 in 2021. This equates to a total decrease of 66% over the 3 years. This reduction of 66% is likely a much more overall accurate reflection of the HO-CDI reduction rate than the 80% reduction calculated from the raw data.
The authors of this manuscript could not provide COHCFA SIR rates because NHSN does not report out CO-HCFA SIR data.
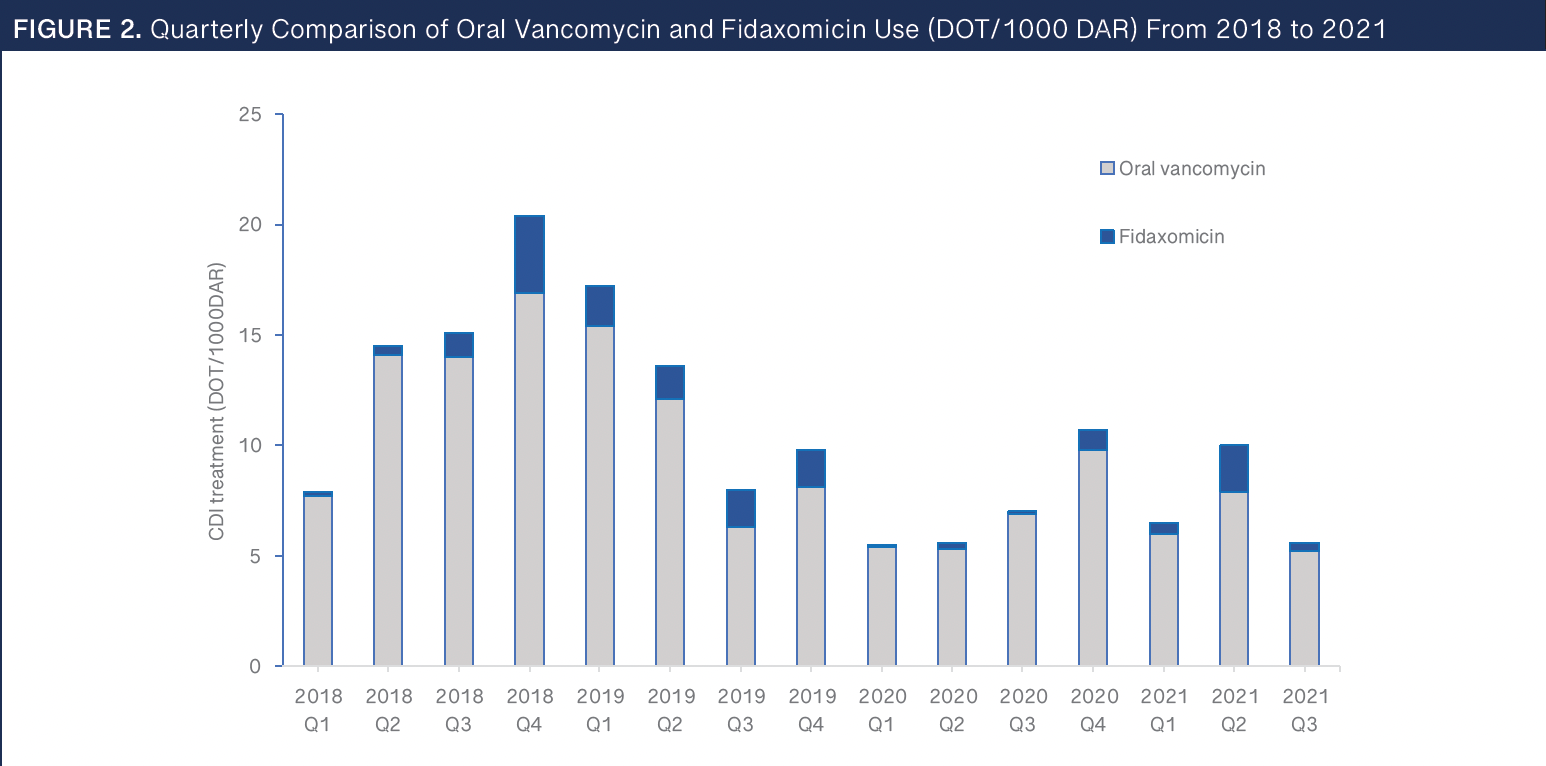
CDI Antimicrobial Rate Trend. To further confirm a reduction in total CDIs, utilization of oral vancomycin and fidaxomicin was assessed. Figure 2 shows the trend of both agents per each quarter from 2018 through quarter 3 of 2021, in DOT/1000 DAR. Data from 2021 are included only through quarter 3 secondary to the hospital’s contract termination with BD MedMined at that time. CDI rates and total oral vancomycin and fidaxomicin use were found to be strongly positively correlated (R = 0.7627).
Fluoroquinolone MUE. Fifty-four patients were assessed in the fluoroquinolone MUE and 43 (80%) were considered to have been “inappropriately” prescribed a fluoroquinolone. Of these 43 patients, it was determined that 13 patients (30%) were unnecessarily prescribed a fluoroquinolone due to a documented β-lactam allergy. Following the 1-month MUE, the AMS pharmacist continued to monitor fluoroquinolone use on most days and real-time feedback and education continued to be provided for providers and pharmacists.
Fluoroquinolone Use vs HO-CDI Rates Comparison. The average usage of fluoroquinolones (including the 2 formulary agents, ciprofloxacin and levofloxacin) for the baseline year 2018 was 74 DOT/1000 DAR, corresponding with 35 HO-CDI cases; in 2019 usage was 46 DOT/1000 DAR, corresponding with 17 HO-CDI cases; and for the study year 2020 usage was 19 DOT/1000 DAR, corresponding with 11 HO-CDI cases. Data from 2021 were also assessed, but only through quarter 3, which is when the hospital’s contract with BD MedMined terminated. Data for the first 3 quarters of 2021 showed an average of 19 fluoroquinolone DOT/1000 DAR, corresponding with 7 HO-CDI cases. Total fluoroquinolone reduction over the 3 years from 2018 to 2021 was 74% (average of 19 vs 74 DOT/1000 DAR). CDI rates and fluoroquinolone use were found to be strongly positively correlated (r = 0.7289). Data for these results are seen in Figure 3.
Discussion
In this retrospective assessment, specific, key pharmacist-led initiatives were thought to have improved outcomes of health care–associated CDIs in a community hospital. To the authors’ knowledge, this is the first analysis to demonstrate multiple, interconnecting pharmacist interventions that may sustainably decrease health care–associated CDIs for a community health system. The aforementioned initiatives on their own likely provided negligible impact on CDI rates; however, it is thought that all the noted initiatives were interconnected and the decline in CDIs may be due to a cumulative effect.
This hospital’s main overutilized antibiotics due to stated penicillin allergies included clindamycin, fluoroquinolones, and carbapenems. After implementation of each of the noted study interventions, the DOT for each of these overutilized antibiotics decreased appreciably. However, it should be noted that this impact did not decrease total organization antibiotic DOT, but rather, it led to a slight overall increase. It was identified that fluoroquinolone use was mainly prescribed for penicillin-allergic patients with a community-acquired pneumonia indication. After the fluoroquinolone initiative, the rate of increase of ceftriaxone use corresponded inversely with the rate of decrease of fluoroquinolones use starting in early to mid-2019. However, this also necessitated the additional use of azithromycin to treat the atypical bacteria in community-acquired pneumonia, thus doubling the DOT rate secondary to the use of 2 agents as opposed to the 1 fluoroquinolone. As carbapenem use declined, cefepime use correspondingly increased. Lastly, clindamycin had previously been overutilized, mainly in the perioperative setting. After β-lactam allergy education and allergy testing initiatives were implemented, clindamycin use in the surgical setting declined by 95%, with a direct corresponding increase in perioperative cefazolin use.
As with any initiative with a noted large impact, it is important to be able to look at long-term sustainability of results. It is noted that in the last 2 quarters of 2020, the total C difficile rates, as well as SIRs, began to trend up. This follows the trends of many HAI increases nationwide due to COVID-19, in part caused by the pulling of resources of AMS and infection prevention teams and other health care staff to focus on the pandemic. However, the AMS pharmacist continued to work throughout the pandemic to note this increase in CDIs. The authors of this study concluded that the hospital’s sustainability may be due to pharmacists not just implementing programs and initiatives to work on issues at the surface, but rather finding the major root of the problem of the prescribing of broad-spectrum and non–β-lactam agent alternatives, which may ultimately be the key to the longevity and sustainability of this program.
Although not study end points, reductions in CDIs both have a positive economic impact and potentially decrease mortality rates, the authors would like to note. Both expected and realized CDI reduction–associated mortality rates were assessed. According to Lessa et al, a population and ab-surveillance study revealed a 30-day observed crude case fatality rate for HO-CDI cases to be approximately 9.3%.25 Mortality attributable directly to CDI was further predicted at 50% of the crude mortality, or a rate of 4.7%. Per the decrease in number of HO-CDI cases from 2018 to 2021, a literature-based assessment estimates approximately 2 all-cause mortality lives saved, or 1 direct C difficile–related life saved, in a 30-day time frame. To calculate a realized or patient-specific reduction in mortality, charts of all HO-CDI cases were reviewed. In 2018, there were 6 all-cause related deaths; in 2019, 3 all-cause related deaths; in 2020, 1 all-cause related death; in 2021, 2 all-cause related deaths. It was noted many of these deaths occurred within 30 days, with 2 outliers within 60 days. It can be presumed that pharmacist-driven initiatives may have contributed to a decrease in all-cause related C difficile mortality cases by 4 total patients within a 3-year time frame. This realized reduction in mortality surpasses the theorized literature-based assessment estimates by 2-fold.
The Agency for Healthcare Research and Quality estimates an average cost savings of $17,260 per C difficile HAI case.26 Extrapolation of this data shows that there was a literature-based cost savings of approximately $483,280 for reduction of HO-CDIs during the 2018 to 2021 time frame. Unfortunately, an actual cost savings could not be determined due to many confounding variables, especially during this study time period, with the impact of COVID-19.
This retrospective analysis does have a few limitations. Primarily, because it was conducted at a 254-bed hospital, the effects are not necessarily generalizable to a larger setting. Other factors that were not mentioned in this analysis but may have had an association with the reduction of C difficile rates include work on the improvement of hand hygiene, the wearing of disposable personal protective equipment, and the addition of bleach products in terminal cleaning in high-risk population rooms. To address other AMS initiatives, it should be noted that CDI rates, in particular with a focus on HO-CDIs, have been closely watched by the AMS program since 2015. From 2015 to 2018, many stewardship initiatives were implemented but without any substantial noted effect on total CDIs. These included an antimicrobial restriction protocol, extended-infusion and dose optimization of select antibiotics, pharmacist-included interdisciplinary rounding, work on adherence to literature-based guidance on the prescribing of antibiotics for community- and hospital-acquired pneumonias, education on limiting total duration of therapy, and education on the testing of, treatment for, and total antibiotic duration of urinary tract infections.
Also important to note, the hospital had pulled together an interdisciplinary C difficile work group at the end of 2018 to brainstorm possible identification of common root causes of previous CDIs and ideas on how to move forward with reducing HO-CDIs. At the end of this work group, there was no identification of key causes of CDIs, although rates were noted to be higher in general on the oncology floor. Plans on moving forward to reduce future CDIs were multifactorial. First, the use of all-bleach wipes for the cleaning of the entire oncology ward was initiated. Second, there was a collaboration with the microbiology lab to change the C difficile multistep testing platform. Previously, the first step utilized was a glutamate dehydrogenase (GDH) enzyme immunoassay (EIA), which uses antibodies to test for the presence of the GDH enzyme, a protein present in all C difficile isolates. The second step in this multistep platform included a reflex for all positives to polymerase chain reaction (PCR)/nucleic acid amplified test, which detects toxigenic C difficile in stool. This platform was updated to a first-step PCR (Focus Diagnostics Simplexa) with reflex to EIA (TechLab C Diff Quik Chek Complete). This new testing allowed for faster and more accurate CDI diagnoses. Third, the antibiotic stewardship program and infection prevention programs included comprehensive education on the appropriate indications for, timing of, and correct testing platform for diarrhea cases. This alone was key to the start of success in reducing CDIs. It was, however, not highlighted in this analysis in part because it was noted by Indiana’s Hospital Improvement Innovation Network Workgroup, a statewide-implemented initiative throughout much of Indiana and the nation (including 1600 hospitals) around the time of the updated change. Changes regionally and nationally drive down the denominator in the equation (O/E) for SIRs, yet this hospital’s SIR continued to drop (by a total of 38% over the 2 years). The authors of this analysis determined that this likely is not the major contributing factor in the success of CDI decreases.
Another important key limitation in this study was the impact of COVID-19. The effects of the pandemic began during the fourth quarter of 2019 and lasted through the rest of the study period. Total hospitalizations declined drastically; total patient hospitalization days at this hospital were down from average rates by 20% in 2020 and remained down by 16% in 2021. Reasons for admissions were changing as well; a decline was seen, for example, in patients with diabetic complications, skin infections, chest pain, elective general surgeries and the typical respiratory illnesses, to instead those with respiratory and other complications due to COVID-19. Antibiotic prescribing changed as well, with increases seen in both total and broad-spectrum antibiotic use to treat possible secondary bacterial infections. Many services were limited, with resources pulled to the pandemic efforts. These changes could have confounding impacts on any study during this time period. However, it should still be noted that the largest reduction in CDI events did occur in quarter 3 of 2019, before the pandemic. As mentioned earlier, CDI and other HAI rates began to increase in the last 2 quarters of 2020, likely due to the pandemic strains on health care systems; however, these rates did again improve in 2021.
One last limitation is that because this research was set in an uncontrolled environment, it is unclear whether any of the interventions provided a direct causal effect in the decline in CDI rates. Because these interventions were interconnected, the authors assume that the interventions provided only a statistical association into decreasing CDI and SIR data. Further data are necessary to determine whether each intervention provides any causal effect in declining CDI trends, potentially with the use of statistical multi-regression testing. Multiregression analysis would allow one to quantify the extent to which interventions independently influenced the observed CDI rate reduction. Moreover, it could elucidate the hypothesized synergistic effects among the interventions and overall offer valuable insight into optimizing future strategies for combating health care–associated CDIs.
The authors of this study welcome other health care facilities of differing locations, sizes, resources, and other factors to work on similar initiatives and report out potential success rates at decreasing CDIs. In the event of limited resources, the authors advise focusing efforts on β-lactam allergy education, which in turn will likely inherently lead to a decline in fluoroquinolone and other broader-spectrum antibiotic usage and ultimately a reduction in CDI rates. It is the hope of the authors of this article that, by sharing this important work, others can emulate similar pharmacist initiatives and help to decrease overall rates of C difficile, and potentially C difficile–associated mortality, nationwide.
Conclusions
Overall, it was found that with the use of local, evidence-based, and antibiogram-supported guidelines, PST, β-lactam allergy education, and decreased fluoroquinolone use, there was an associated decrease in total CDIs at this community hospital. As noted above, there was a total 3-year decrease in HO-CDI rates of 80%, and a 3-year decrease in CO-CHFA-CDI rates of 81%. There was a corresponding HO-CDI SIR reduction of 66%. There was also a potential realized decrease in C difficile–associated all-cause mortality by 4 patients within a 3-year time frame. Furthermore, there was a proven sustainability seen by looking at data 2 years post-initiatives’ analysis.
References
Czepiel J, Dróżdż M, Pituch H, et al. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38(7):1211-1221. doi:10.1007/s10096-019-03539-6
Marra AR, Perencevich EN, Nelson RE, et al. Incidence and outcomes associated with Clostridium difficile infections: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(1):e1917597. doi:10.1001/jamanetworkopen.2019.17597
2019 AR threats report. CDC. Reviewed November 23, 2021. Accessed February 3, 2024. https://www.cdc.gov/drugresistance/biggest-threats.html
Healthcare-associated infections. CDC. Reviewed November 10, 2021. Accessed February 3, 2024. https://www.cdc.gov/hai/dpks/deadly-diarrhea/dpk-deadly-diarrhea.html
Wenisch JM, Equiluz-Bruck S, Fudel M, et al. Decreasing Clostridium difficile infections by an antimicrobial stewardship program that reduces moxifloxacin use. Antimicrob Agents Chemother. 2014;58(9):5079-5083. doi:10.1128/AAC.03006-14
Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(7):1748-1754. doi:10.1093/jac/dku046
Bower D, Hachborn F, Huffam P. Clostridium difficile outbreak: a small group of pharmacists makes a big impact. Can J Hosp Pharm. 2009;62(2):142-147. doi:10.4212/cjhp.v62i2.443
DiDiodato G, McArthur L. Evaluating the effectiveness of an antimicrobial stewardship program on reducing the incidence rate of healthcare-associated Clostridium difficile infection: a non-randomized, stepped wedge, single-site, observational study. PLoS One. 2016;11(6):e0157671. doi:10.1371/journal.pone.0157671
Patton A, Davey P, Harbarth S, Nathwani D, Sneddon J, Marwick CA. Impact of antimicrobial stewardship interventions on Clostridium difficile infection and clinical outcomes: segmented regression analyses. J Antimicrob Chemother. 2017;73(2):517-526. doi:10.1093/jac/dkx413
Mijović B, Dubravac Tanasković M, Račić M, Bojanić J, Stanić S, Banković Lazarević D. Outcomes of intrahospital antimicrobial stewardship programs related to prevention of Clostridium difficile infection outbreaks. Med Glas (Zenica). 2018;15(2):122-131. doi:10.17392/958-18
Wenzler E, Mulugeta SG, Danziger LH. The antimicrobial stewardship approach to combating Clostridium difficile. Antibiotics (Basel). 2015;4(2):198-215. doi:10.3390/antibiotics4020198
Macy E, Khan DA, Castells MC, et al. Penicillin allergy testing: A key component of antibiotic stewardship. Clin Infect Dis. 2017;64(4):531-532. doi:10.1093/cid/ciw795
Gugkaeva Z, Crago JS, Yasnogorodsky M. Next step in antibiotic stewardship: pharmacist-provided penicillin allergy testing. J Clin Pharm Ther. 2017;42(4):509-512.doi:10.1111/jcpt.12530
Solensky R, Khan DA, Bernstein IL, et al; Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. doi:10.1016/j.anai.2010.08.002
Macy E. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep. 2014;14(11):476. doi:10.1007/s11882-014-0476-y
Zagursky RJ, Pichichero ME. Cross-reactivity in β-lactam allergy. J Allergy Clin Immunol Pract. 2018;6(1):72-81.e1. doi:10.1016/j.jaip.2017.08.027
Romano A, Valluzzi RL, Caruso C, Maggioletti M, Quaratino D, Gaeta F. Cross-reactivity and tolerability of cephalosporins in patients with IGE-mediated hypersensitivity to penicillins. J Allergy Clin Immunol Pract. 2018;6(5):1662-1672. doi:10.1016/j.jaip.2018.01.020
Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188-199. doi:10.1001/jama.2018.19283
Hester SA. Allergic cross-reactivity among beta-lactam antibiotics. RL Andrews. October 2013. Accessed November 30, 2023. https://www.rlandrews.org/pdf_files/cross_reactivity/cephalosporin_cross_reactivity.pdf
Trubiano JA, Chen C, Cheng AC, et al. Antimicrobial allergy ‘labels’ drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother. 2016;71(6):1715-1722. doi:10.1093/jac/dkw008
Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
Jones BM, Bland CM. Penicillin skin testing as an antimicrobial stewardship initiative. Am J Health Syst Pharm. 2017;74(4):232-237. doi:10.2146/ajhp160233
Fatima R, Aziz M. The hypervirulent strain of Clostridium difficile: NAP1/B1/027 - a brief overview. Cureus. 2019;11(1):e3977. doi:10.7759/cureus.3977
Valiente E, Cairns MD, Wren BW. The Clostridium difficile PCR ribotype 027 lineage: a pathogen on the move. Clin Microbiol Infect Dis. 2014;20(5):396-404. doi:10.1111/1469-0691.12619
Lessa FC, Mu Y, Bamberg WM. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834. doi:10.1056/NEJMoa1408913
Estimating the additional hospital inpatient cost and mortality associated with selected hospital-acquired conditions. Agency for Healthcare Research and Quality. Reviewed November 2017. Accessed November 30, 2023. https://www.ahrq.gov/hai/pfp/haccost2017-results.html
Disclosure: The authors declare no conflicts of interest. No funding sources exist.