News
Article
FDA Approves Interchangeable Biosimilar Ustekinumab-auub for Inflammatory Diseases
Author(s):
The biosimilar, now legally available at the pharmacy level, demonstrated similar safety, purity, and potency to the reference product.
Ustekinumab-auub (Wezlana; Amgen) has received FDA approval as a biosimilar to ustekinumab (Stelara; Janssen) for the treatment of inflammatory diseases. Concurrently, the FDA approved ustekinumab-auub (ustekinumab) as an interchangeable biosimilar with ustekinumab, with the biosimilar showing no clinically significant differences in safety and efficacy for the indicated conditions. The approval potentially allows a more cost-effective product to come to market, according to FDA officials in a recent statement.
image credit: SergeVo | stock.adobe.com
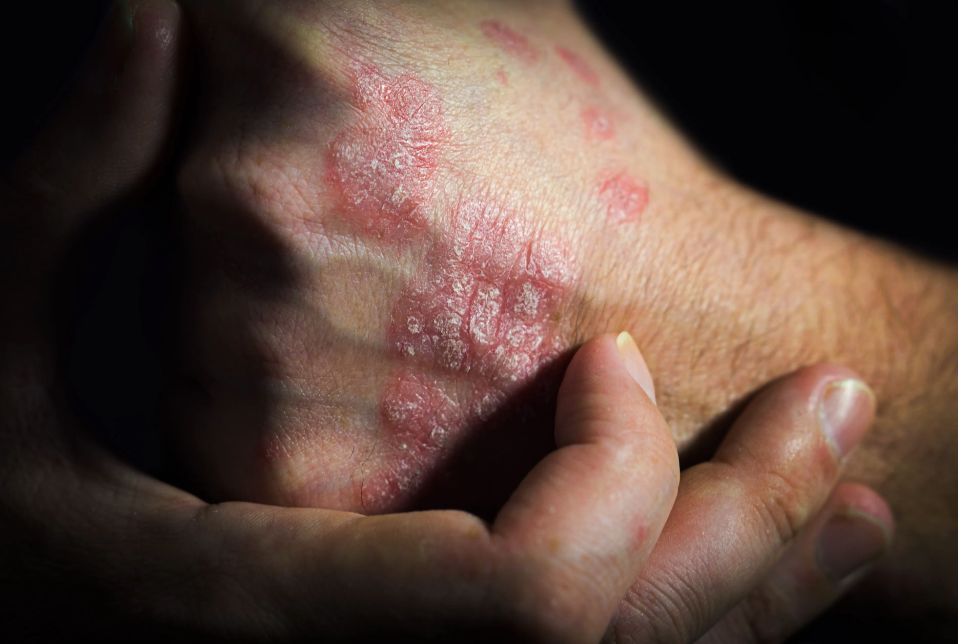
“This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost,” said Sarah Yim, MD, FDA, director of the Office of Therapeutic Biologics and Biosimilars in the Center for Drug Evaluation and Research, in the statement.
Ustekinumab is indicated for the treatment of moderate to severe plaque psoriasis in adult patients who qualify for phototherapy or systemic therapy; active psoriatic arthritis; moderately to severely active Crohn disease; and moderately to severely active ulcerative colitis in adult patients. Among pediatric patients aged 6 years and older, ustekinumab is indicated for the treatment of plaque psoriasis among those who qualify for phototherapy or systemic therapy, and active psoriatic arthritis.
Biosimilars are biologic products that have a strong similarity to, but are not clinically different from, current FDA-approved biological products. The FDA’s interchangeable designation for biosimilars allows pharmacists to make substitutions for a reference product without the need for a prescription. When an interchangeable biosimilar meets its lawful requirements (be it state pharmacy laws or others) it can be made available at certain pharmacies who make a pharmacy-level substitution of the biosimilar in place of the brand-name drug.
Prior to receiving FDA approval, the interchangeable drug went through a series of chemical tests and biological tests and assays to confirm that it shared structural and functional features with the reference product (Stelara; Janssen). Investigators also gathered evidence from comparative human pharmacokinetic data, clinical immunogenicity data, and safety and efficacy data, which demonstrates that both products share clinically meaningful similarity in safety, purity, and potency.
The most common adverse events (AEs) associated with ustekinumab include nasopharyngitis, upper respiratory tract infection, headache, fatigue, nausea, vomiting, injection site erythema, vulvovaginal candidiasis/mycotic infection, bronchitis, pruritus, urinary tract infection, sinusitis, abdominal pain, influenza, fever, and diarrhea. Infection is listed as a severe AE that is known to occur, the FDA wrote in the statement.
The product will contain labeling that discusses risks and other AEs, and an associated Medication Guide will break down these risks and use information in more detail.
“Biosimilar medications offer additional safe and effective treatment options that have the potential to increase access for people requiring treatment for inflammatory diseases,” said Nikolay Nikolov, MD, director of the Office of Immunology and Inflammation in the FDA’s Center for Drug Evaluation and Research, in the statement. “Today’s approval could have a meaningful impact for patients managing their disease.”
Reference
FDA Approves Interchangeable Biosimilar for Multiple Inflammatory Diseases. FDA. News Release. October 31, 2023. Accessed on November 1, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-interchangeable-biosimilar-multiple-inflammatory-diseases
Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.






