
Comprehensive discussion on the identification of Clostridioides difficile infection, the spectrum of disease, and overall epidemiology.

Comprehensive discussion on the identification of Clostridioides difficile infection, the spectrum of disease, and overall epidemiology.

A broad review of BTK inhibition as a novel therapeutic approach toward the treatment of marginal zone lymphoma.

The panel reflects on how disruption of the gut microbiome leads to the proliferation of Clostridioides difficile.

Following discussion on the NCCN guidelines for second-line therapy in marginal zone lymphoma, experts highlight approved treatment options.

Ray Tancredi, RPh, MBA, divisional VP of specialty pharmacy development and brand Rx/vaccine purchasing at Walgreens, provides an analysis and overview of key specialty pharmaceuticals in development.
Ismail Lourido Ali, the director and counsel of policy and advocacy at the Multidisciplinary Association for Psychedelic Studies (MAPS), discusses continuing education opportunities for pharmacists to get trained on the proper use and administration of psychedelic drugs.
Ron Aung-Din, MD, the clinical medical advisor of Pyscheceutical, and Chad Harman, the CEO of Pyscheceutical, discuss a novel approach for administering psychedelics for the treatment of brain disorders that does not induce hallucinogenic effects.

Ray Tancredi, RPh, MBA, divisional VP of specialty pharmacy development and brand Rx/vaccine purchasing at Walgreens, discusses key drugs in development that are slated to launch in 2022.

Joanna Lewis, PharmD, MBA, presents data from the Pharmacy Times’ OTC Guide and explains the pharmacist recommended options for over-the-counter immune support supplements.

Joanna Lewis, PharmD, MBA, gives an overview of eczema and its symptoms and provides details on the best available over-the-counter medications.

Rita Wise, DEd, MSN, RN, program director of the Masters in Nursing Education and Nursing Administration programs at Pennsylvania College of Health Sciences, discusses her hopes for how the country and health systems will respond to the national nurse staffing crisis.

Minority researchers and investigators from underrepresented communities often have access to less clinical research funding than other clinical researchers.

Joanna Lewis, PharmD, MBA, discusses practical considerations when selecting over-the-counter medications to treat common cough, cold and flu symptoms.

Shared insight on treating marginal zone lymphoma with the current treatment armamentarium.

Shared insight on how disruption of the gut microbiome may occur based on antibiotic exposure, environmental factors, and diet.

Expert panelists review the pathophysiology of marginal zone lymphoma and reflect on its prevalence as a rare disease.

Opening their discussion on Clostridioides difficile, experts provide an overview on the gut microbiome and colonization resistance.

Social determinants of health can play a significant role in the treatment trajectory and outcomes for patients with cancer because oncology care requires close communication between the patient and clinicians.
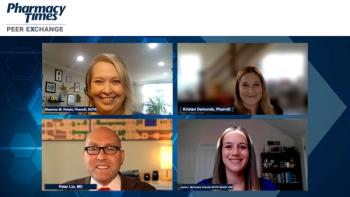
Kristen Demundo, PharmD, and Shannon M. Rotolo, PharmD, BCPS, review the important role pharmacists play in helping patients manage their atopic dermatitis condition and treatment.

A panel of experts discuss JAK inhibitors and their potential impact on the management of atopic dermatitis.

Pharmacy Times spoke to Michael Young, MBA, Vice President of Pharmacy Strategy, Parata Systems, about centralization and automation in the pharmacy field and how these tools can create new solutions for pharmacists.
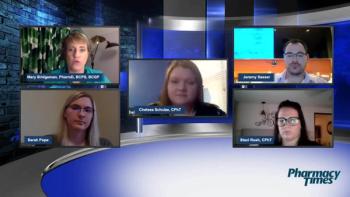
Panel of experts discuss the increasing roles and responsibilities in the pharmacy technician space as integral members of the pharmacy patient care team.

Pharmacists should keep an eye on the pipeline for autologous and allogeneic CAR T-cell therapies as well as antibody-specific treatments.

Joanna Lewis, PharmD, MBA, highlights data from Pharmacy Times®’ OTC Guide and discusses practical information on the selection of sunscreen to prevent skin damage and cancer.
Hizentra is indicated for the treatment of primary immunodeficiency in adults and pediatric patients 2 years of age and older and maintenance therapy in adults with chronic inflammatory demyelinating polyneuropathy.

With so many approved regimens and common use of oral agents in the treatment of multiple myeloma, oncology pharmacists are essential team members.
Gaby Gabriel, MD, program director, interventional radiology residency and assistant program director, diagnostic radiology residency, University of Kentucky, discusses the implications of research into the treatment of liver metastases in patients with gastrointestinal NETs.
Adam Olszewski, MD, associate professor of medicine in Alpert Medical School at Brown University, discusses the role setting has in the care of patients with Burkitt lymphoma.

Kyle Detwiler, CEO of Clever Leaves, discusses his company’s donation of $1 million in medical-grade cannabis to research organizations for the purposes of advancing medical research.

Gaby Gabriel, MD, program director, interventional radiology residency and assistant program director, diagnostic radiology residency, University of Kentucky, discusses the findings regarding the efficacy of combining and sequencing liver-directed therapy with systemic therapy.