
Enfortumab vedotin is being investigated for the treatment of locally advanced or metastatic urothelial cancer following both platinum-containing chemotherapy and a PD-1 or PD-L1 inhibitor.

Enfortumab vedotin is being investigated for the treatment of locally advanced or metastatic urothelial cancer following both platinum-containing chemotherapy and a PD-1 or PD-L1 inhibitor.

Top news of the day from across the health care landscape.

Top news of the week from Specialty Pharmacy Times.

The CDC's Tips From Former Smokers campaign has new powerful messages to encourage individuals to quit smoking.

For the study, the researchers assessed whether a slow-release, long-acting, implantable naltrexone could improve outcomes for patients with HIV and opioid addiction.

The matching of optimized technologies and novel approaches has provided investigators with a framework to assess and understand neurologic conditions.

The announcement follows months of investigating impurities found in ARBs.

The indication�’s expansion for palbociclib to include male patients is based upon data from postmarketing reports and electronic health records showing that the safety profile for men treated with the drug is consistent with the safety profile in women treated with this medication.

By participating in the P&T Competition, student pharmacists gain experience in gathering and critically analyzing scientific, clinical, and economic evidence in a systematic and rigorous fashion, according to the foundation.
Specialty pharmacy can benefit from having definitive a center of excellence model that caters to respective therapies and disease states.

An implantable form of naltrexone was more effective in improving HIV outcomes in patients compared with oral naltrexone.

Top news of the day from across the health care landscape.

Rizaport is an oral film formulation of rizatriptan benzoate, the active drug in Maxalt, for patients with migraine who experience dysphagia or migraine-related nausea.
The hearing will take place May 13, 2019 at the agency’s headquarters in Silver Spring, Maryland.

Melatoin is an antioxidant and stimulates growth receptors in the skin and hair.

Voluntary reports indicate that some individuals, especially youth and young adults, are experiencing seizures or convulsions following use of e-cigarettes, although a definitive link has not been established.
The daratumumab (Darzalex) combination therapy would be the first for use in the frontline setting for transplant-eligible patients with multiple myeloma.

Top news of the day from across the health care landscape.

HIV infection is associated with higher rates of hepatitis C replication.

If approved, Gimoti would be the first non-oral drug treatment for symptoms associated with acute and recurrent diabetic gastroparesis in adult women and would represent the first significant advancement in the treatment of gastroparesis in 40 years.
The warning letters issued to azmedicinalshop.com and thedonrx.net request that these enterprises immediately cease offering violative drugs for sale to consumers in the United States.

Immune Globulin Intravenous, Human – slra 10% Liquid (Asceniv) is indicated for use in the treatment of primary humoral immunodeficiency disease in adults and adolescents.
Although lung cancer screenings are covered at no cost by most insurers, state Medicaid programs are not required to offer coverage for this service.

Immune Globulin Intravenous, Human – slra 10% Liquid (Asceniv) is indicated for use in the treatment of primary humoral immunodeficiency disease in adults and adolescents.

Top news of the day from across the health care industry.
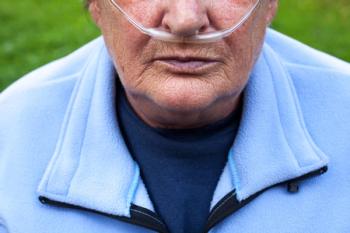
The combination treatment (Duaklir, Circassia) is designed to be administered twice-daily via AstraZeneca’s breath-actuated inhaler (Pressair).

The combination therapy was approved for twice-daily administration via the breath-actuated Pressair inhaler in patients with COPD.

Avaclyr 3%, a herpes simplex virus nucleoside analog DNA polymerase inhibitor.

In addition to concerns about contamination, some products made by these companies that are labeled as homeopathic do not deliver any benefit, yet may potentially be harmful, according to the FDA.

Gilteritinib (Xospata) was superior to standard chemotherapy in improving overall survival in patients with acute myeloid leukemia.