
With rapid growth, health system specialty pharmacies are more than likely to run into several operational pain points.

With rapid growth, health system specialty pharmacies are more than likely to run into several operational pain points.

Top news of the week from Specialty Pharmacy Times.

Top news of the day from across the health care landscape.

Pembrolizumab (Keytruda, Merck) plus chemotherapy met the primary endpoint of pathological complete response in patients with triple-negative breast cancer in a phase 3 study.

The findings represent the first positive phase 2b study of a personalized cancer vaccine in patients with high-risk melanoma.

With specialty pharmacy emerging as the fastest growing segment of the pharmacy industry, health systems have to address a key question: can you scale for it?

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

Announced by the HHS and FDA, the plan seeks to safely import medications that would provide a cost savings to consumers, but some pharmacy and other health care professionals have expressed concerns.

Study provides the longest available follow-up on the survival of patients with advanced melanoma, renal cell carcinoma, and non-small cell lung cancer treated with nivolumab.

About 1000 patients are helped each month to reduce their costs for medications through the initiative.
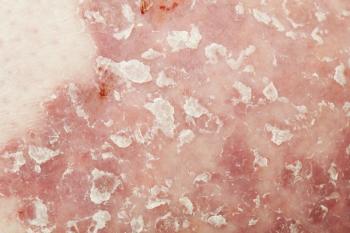
Officials with the FDA have taken action on LEO Pharma’s calcipotriene and betamethasone dipropionate foam and topical suspension products for the topical treatment of plaque psoriasis in patients age 12 years and older.

Ashlee Klevens Hayes is a pharmacist, a keynote speaker, a co-host on the Pharmacy Podcast Network with her show Rx Buzz, and an entrepreneur with her business Rx Ashlee.

Darolutamide is an androgen receptor inhibitor (ARi) for the treatment of patients with non-metastatic castration-resistant prostate cancer.
Abemaciclib (Verzenio, Eli Lilly) in combination with fulvestrant demonstrated a statistically significant improvement in overall survival among women with metastatic breast cancer previously treated with endocrine therapy.

Top news of the day from across the health care landscape.

The American Society of Health-System Pharmacists (ASHP) has released a statement opposing the Centers for Medicare and Medicaid Services’ (CMS) proposed cuts to reimbursement for drug purchased through the 340B Drug Pricing Program.
Vaccine found to provide durable protection against the “death star†HIV strain, which is notably hard to fight.

Darolutamide, an androgen receptor inhibitor (ARi) for the treatment of patients with non-metastatic castration-resistant prostate cancer, was approved by the FDA this week.

The new product is available in the United States in 20- and 30-count sizes.

Researchers aimed to determine the potential pathology of encephalitis as a complication of immunotherapy treatment.
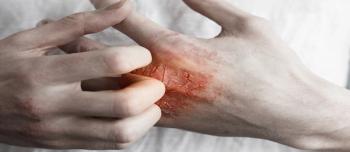
Top news of the day from across the health care landscape.

The MJH SAP program partners Hematology-Oncology Associates of Central New York with Targeted Oncology™, The American Journal of Managed Care® and Pharmacy Times®,

Mylan and Pfizer have announced an agreement to combine Mylan with Upjohn, Pfizer’s off-patent branded and generic established medicines business.

Data from a pair of phase 3 trials were recently presented at the 10th International AIDS Society Conference on HIV Science (IAS 2019) in Mexico City.

Erdafitinib (Balversa, Janssen) is the first FGFR inhibitor to receive FDA approval for the treatment of patients with metastatic bladder cancer marked by FGFR gene mutations.

Why is this healthy young man getting severe brain fog?

Top news of the day from across the health care landscape.
Administered through a drug-eluting implant, islatravir demonstrated protection against HIV for up to 12 months.
In a study by Johns Hopkins Medicine, investigators used an advanced form of brain scan to demonstrate that 12 participants in their study with documented posttreatment Lyme disease syndrom (PTLDS) all showed elevation of a chemical marker of wide-spread brain inflammation compared with 19 controls.