The combination of COVID-19 with opioid-induced respiratory depression, especially in an elderly patient, could be deadly.

The combination of COVID-19 with opioid-induced respiratory depression, especially in an elderly patient, could be deadly.

In the digital age, exponential technologies are converging and transforming how we engage in health care.

Device uses an Apple Watch to deliver gentle vibrations to users suffering from post-traumatic stress disorder.
Researchers found that COVID-19 severity was linked to cardiovascular risk factors, such as obesity, high blood pressure, diabetes, and high cholesterol.

The FDA has granted a breakthrough therapy designation to zanidatamab for the treatment of patients with HER2 gene–amplified biliary tract cancer who have received prior therapy.
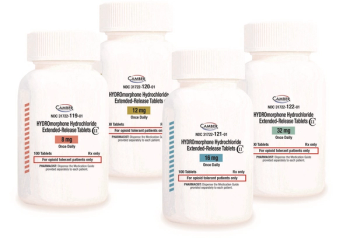
Camber Pharmaceuticals is pleased to announce that it has launched Hydromorphone ER tablets, which is the generic version of Exalgo.
The new optical sensing method is based on multi-pass spectroscopy and can detect the presence of 1 molecule of formaldehyde in a million air particles, or 1 part per million, even in the presence of gasses that often interfere with optical measurements.

Students answer the question: How have campus groups or pharmacy organizations remained active during the pandemic?

Even when all 5 factors were optimally controlled, patients with type 2 diabetes still had a 21% higher risk for CVD and a 31% higher risk for heart failure hospitalization compared to patients without diabetes.

Pharmacy Times spoke with Angela Kashuba, Dean of UNC Eshelman School of Pharmacy and Patrick Brown, UNC Eshelman School of Pharmacy Alumni, about preparation for the possibility of a general public COVID-19 vaccine.

Moderna intends to submit the EUA application today and believes that the FDA’s Vaccines and Related Biological Products Advisory Committee will meet to review the safety and efficacy data package on Thursday, December 17, according to the press release.

This week on Pharmacy Times, there are a number of important topics that will be covered and posted throughout the week.
Patients with COVID-19 faced a significant emotional and financial burden after discharge from treatment.

Investigators found that participants with the healthiest sleep habits had a 42% reduction in the risk of heart failure compared to participants with an unhealthy sleep pattern.

Smart inhalers have the potential to address the challenges faced by patients and health care professionals in the management of respiratory diseases.

Most web searches for insomnia were done between midnight and 5 am, when people are typically asleep.

The study showed that 95% of women who tested positive for COVID-19 during pregnancy had no adverse outcomes, with the virus being transmitted to the fetus in just 3% of the cases.

Although cancer deaths with the highest death rates per capita accounted for the greatest number of years lost, the study authors said cancers that typically occur in younger populations had a disproportionate share of the burden.

Pharmacy Times interviewed Rick Miller, the vice president of clinical and professional services at Alliance RX Walgreens Prime to talked about the changing specialty pharmacy field.

In an interview with Pharmacy Times, Peter Bonis, MD, chief medical officer of clinical effectiveness at Wolters Kluwer Health, said formulary adherence in health systems is essential for several reasons.

Examining several biomarkers to look for biological explanations for these results, the research team found key mechanisms, including insulin resistance, body mass index (BMI), lipoprotein metabolism, and inflammation, according to the study authors.

In an interview with Pharmacy Times, Peter Bonis, MD, chief medical officer of clinical effectiveness at Wolters Kluwer Health, said collaboration between pharmacists, physicians, and other health care workers is essential.

According to the study results, women interested in medical cannabis reported marginally lower levels of support from their primary care provider and significantly less support from specialist physicians.

The authors noted that if regulatory agencies maintain the well-documented safety testing protocols that have been developed over the years, COVID-19 vaccines will be safe and trustworthy.

Originally approved in 2018, baloxavir marboxil is indicated for patients aged 12 years and older.
By volunteering with the American Red Cross, pharmacists can continue to help support communities who are struggling during the coronavirus disease 2019 (COVID-19) pandemic.

In the midst of a pandemic, visibility and interoperability have never been more critical when it comes to medication inventory.
This week on Pharmacy Times, there are a number of important topics that will be covered and posted throughout the week.
To analyze digital images of metastatic tumors of melanoma, the researchers used computer algorithms, or deep convolutional neural networks (DCCN), to identify patterns associated with treatment response.

It can be administered as a fixed-dose formulation over approximately 3 to 5 minutes, which is significantly less time than the intravenous (IV) formulation.