
Christopher Herndon, PharmD, BCACP, FASHP, FCCP, CPE, discusses the upcoming BPS pain management certification launching in late 2025, its role in validating pharmacist expertise.

Christopher Herndon, PharmD, BCACP, FASHP, FCCP, CPE, discusses the upcoming BPS pain management certification launching in late 2025, its role in validating pharmacist expertise.
Data from the CodeBreaK 300 trial supported the FDA's approval.

Pharmacies are actively working to address issues and support their patients by collaborative with regulatory bodies like the Board of Pharmacy, and their engagement with the local community.
Acalabrutinib with bendamustine and rituximab shows efficacy in patients with untreated mantel cell lymphoma by increasing progression-free survival in the ECHO trial.
As per the Inflation Reduction Act, the negotiations with the drug companies of the 15 drugs will occur in 2025, with the negotiated prices going into effect in 2027.
Hormonal contraceptives did not increase breast cancer risk in patients with BRCA2 mutations.
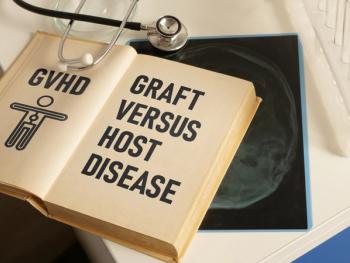
The current approved dose of axatilimab-csfr is 0.3 mg/kg up to a maximum dose of 35 mg and will be administered as an intravenous infusion over 30 minutes every 2 weeks.
This is mirikizumab’s second FDA-approved indication in inflammatory bowel disease.

Rite Aid survey highlights underutilized role of pharmacists in health care, with 62% of respondents viewing their pharmacists as a crucial part of their health and wellness care team.
A politically and economically stressed system of health care delivery and benefits is manifesting in the pharmacy benefit management sector.

Farah Towfic, PharmD, MBA, RPh, discusses how US Pharmacopeia helped to strengthen supply chain resilience in 2024 through data-driven insights, policy advocacy, and pharmacist engagement to address prolonged drug shortages, particularly for generic sterile injectables.
The combination yields promising results but was associated with high incidence of toxicity and infection.

Pharmacists help patients manage allergies and asthma by identifying triggers, recommending appropriate treatments, ensuring proper medication usage, and finding affordable options.
However, investigators also find that the combination did not meet the primary end point of increasing progression free survival.

The finding could help better treat patients with long COVID, as pharmacists and treatment providers can look out for ME/CFS symptoms.



Judith Alberto, MHA, RPh, BCOP, director of clinical initiatives at Community Oncology Alliance, discusses key policy issues affecting community oncology pharmacists in 2025.

The test meets a critical unmet need for patients with Alzheimer disease who have not yet been undiagnosed.
Studies shows that Red Dye No. 3 was associated with tumor growth in male rats.

Radon is a tasteless, odorless radioactive gas associated with DNA damage and high genomic tumor instability.

Jim Dente of Advanced Pharmacy Solutions emphasizes the growing role of automation, artificial intelligence, and patient-centric business models in streamlining operations, improving patient care, and addressing industry challenges such as staff burnout and medication errors.

mRNA-1345 (mRESVIA; Moderna) has the potential to significantly transform the RSV disease state in 2025, according to data analysts.
Severe alcohol-associated hepatitis has limited effective pharmacological therapies, emphasizing the need for treatment options.
Posttraumatic stress disorder is found to be associated with coronary artery disease, hypertension, and heart failure.
The findings suggest that patients with alopecia areata (AA) who also have asthma may benefit from increased clinical monitoring and earlier intervention.
Patients with methotrexate (MTX)-induced acute kidney injury treated with glucarpidase were 2.70-times more likely to have kidney recovery and recover faster.
The approach favors administration of frequent low-dose chemotherapy to reduce toxicities and optimize outcomes.

Building on a previous report that revealed corrupt business practices among the big 3 PBMs, a second interim report finds that PBMs heightened prices for important specialty generic drugs to increase their profit margins.