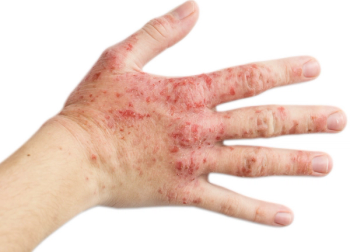
Tralokinumab-ldrm is an interleukin-13 antagonist recently approved for the treatment of moderate-to-severe atopic dermatitis.

Tralokinumab-ldrm is an interleukin-13 antagonist recently approved for the treatment of moderate-to-severe atopic dermatitis.

Ixazomib (Ninlaro, Takeda) has been found to improve survival in patients with multiple myeloma who have not undergone autologous stem cell transplant.

Sagely Naturals Pain Relief Cream combines CBD with powerful botanicals, peppermint, and menthol.

Diroximel fumarate (Vumerity) is indicated for the treatment of relapsing forms of multiple sclerosis.

Pharmacists are taking advantage of new opportunities, but there remain many other opportunities yet to be seized.
The calculation instrument displayed good discrimination, with a C-index of 0.72, as well as good calibration, investigators say.

Data suggest that omicron-specific CD8-positive T cell responses were more than 80% cross-reactive with the CD8-positive T cell response to the original strain of the virus.

New study results show that rapidly adding pounds in the first and final months of gestation likely plays a key role in the development of excess fat tissue in female children and adolescents.

Larotrectinib was the first drug to receive accelerated FDA approval for patients of all ages based on a common molecular marker, independent of tissue origin for targeted therapy.

Applying these findings in practice could improve clinicians’ ability to offer truly personalized cancer therapy by enabling consideration of toxicity along with other data predicting patients’ responses.

A product containing phosphoric acid as well as alcohol successfully neutralized all of the virus particles.
Fecal microbiota transplantation produces cure rates of 82%-88% in patients with recurrent clostridium difficile infections.
Selinexor demonstrated a 37% increase in probability that patients treated with the drug will be in remission at 12 months.

The use of a unique trimer to produce tier-2 neutralizing antibodies may help fight HIV.

Vazalore has been shown to cause fewer stomach-related adverse effects than traditional aspirin.
Pain from golfer's elbow may spread into the forearm and wrist.

Sutimlimab-jome is the only approved treatment for individuals with CAD and works by inhibiting the destruction of red blood cells.

The uncommon disease shares features of both lymphoma and myeloma.

The majority of study participants demonstrated improvement of infraorbital hollows through 1 year.

The agency has also approved the baclofen oral suspension for Azurity Pharmaceuticals to treat individuals with other spinal cord diseases or injuries.
Following the first 3 days after a COVID-19 diagnosis, the risk of stroke quickly decreased.

Data have shown that patients with treatment-resistant anxiety, depression, and post-traumatic stress disorder are among those who would benefit from an expanded treatment offering that would include psychedelic medicines.
Monoclonal antibodies are highly effective at treating the virus, but they have often gone to the healthiest patients, instead of those over aged 65 years.
PainBloc inhibits joint pain from arthritis at the source.
Culturally tailored patient navigation can improve underserved individuals’ health literacy, trust in the health care system, and use of cancer screening tools, study results show.
Ten quiz questions to assess your knowledge on common symptoms and treatments for psoriasis.

Growing pains may be linked to a lowered pain threshold or, in some cases, to psychological issues.

The results of a pilot program investigating the efficacy of remote patient monitoring for patients with cancer showed that it helped to keep high-risk individuals out of the hospital.

Communication, flexibility, and virtual solutions will remain key as the COVID-19 pandemic continues.

Effective specialty pharmacies learn to meet the changing needs of patients and stakeholders.