The results displayed well tolerability and safety with Abrysvo, meeting the study’s co-primary endpoint of immunogenicity and primary safety

The results displayed well tolerability and safety with Abrysvo, meeting the study’s co-primary endpoint of immunogenicity and primary safety

Adrijana Kekic, PharmD, a pharmacogenomics clinical specialist with Mayo Clinic in Phoenix, Arizona, discussed current guidelines for pharmacogenomics.

With the first FDA-approved option hitting shelves soon, pharmacists can advise patients on the best methods to prevent pregnancy

Through recognizing the importance of research in the scope of pharmacy practice by national pharmacy organizations, research can become a prominent role and responsibility of pharmacy.

Eli Lilly and Company will submit results of the study to support tirzepatide (Zepbound) for treatment of obstructive sleep apnea later this year.
Enfortumab vedotin in combination with pembrolizumab showed a manageable safety profile and promising progression free survival and overall survival for locally advanced or metastatic urothelial cancer.
The product is currently being investigated alongside TLX101 in the IPAX-Linz and IPAX-2 clinical trials, and have previously shown efficacy in the IPAX-1 trial.

Pharmacists can play important roles in counseling patients and, in some states, prescribing contraception.
IMPACT CKD, a modeling analysis created by AstraZeneca, includes data about chronic kidney disease across 8 countries, including the United States.
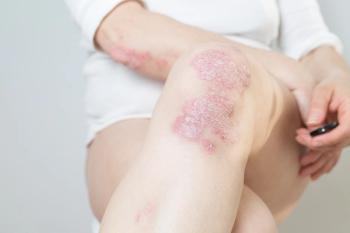
Ustekinumab (Stelara; Janssen Immunology) is a human monoclonal antibody that treats immune-mediated diseases such as psoriasis and psoriatic arthritis.

Access to birth control is a crucial part of preventive health care, but financial barriers persist

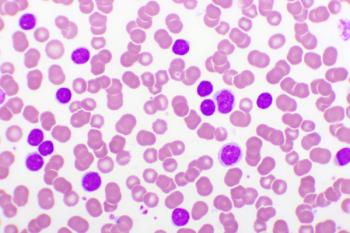
This review discusses the prevalence, mechanisms of development, and evolving treatment landscape in chronic lymphocytic leukemia.
If approved, the test could provide more timely and accurate diagnosis, hopefully mitigating the impact of Alzheimer disease (AD) on individuals and the community.
AI offers many benefits in health professions by efficiently handling time-consuming tasks crucial for patient care.

The study is the first of 5 studies co-published by AYR Wellness and LECOM and showcases improvements in physical, social, and mental well-being with minimal AEs.

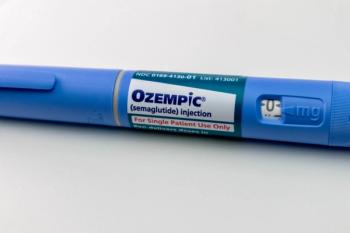
The latest FDA approval paves the way for expanded use of GLP-1 receptor agonists in patients without diabetes.

The extension trial of atogepant demonstrated an 8.5-day improvement of monthly migraine days at weeks 13 through 16 and no new safety signals were observed.

The 5-in-1 meningococcal ABCWY vaccine candidate has an assigned Prescription Drug User Fee Act action date of February 14, 2025.
Erectile dysfunction medications started the shift, then coinsurance, deductibles, and the COVID-19 pandemic accelerated it.
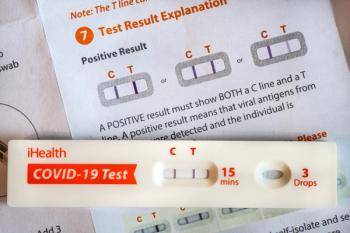
The authors note that additional research needs to be conducted to confirm whether the findings are consistent in adult patients.

Sa’ed Al-Olimat, PharmD, co-founder of the Psychedelic Pharmacists Association, discussed the future of psychedelic medicines and the unique ethical challenges they present.

The campaign for National Stress Awareness Month in April is under way.
Danielle Roman, PharmD, BCOP, discusses the efficacy and safety outcomes of CDK4/6 inhibitors in early-stage breast cancer, as well as key considerations for CDK4/6 inhibitors in this indication.

Kirollos Hanna, PharmD, BCPS, BCOP, FACCC, addresses some common challenges pharmacists may be facing when looking to operationalize bispecifics at their facilities.
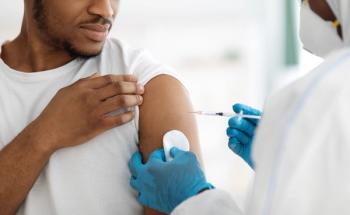
Investigators also found that the pneumococcal conjugate vaccine 13 protected against nasopharyngeal carriage in children and provided some indirect protection for adults.
Although the vaccine has not yet been tested in humans, it demonstrated 100% efficacy in protecting primates who were injected with human Marburg virus disease.
There was no difference observed between adalimumab (Humira; AbbVie) and adalimumab-aacf (Idacio; Fresenius Kabi) for those who switched to the biosimilar.

Investigators found that the individuals’ peak cognitive performance coincided with glucose levels that were slightly above the normal range.
SGLT-2 inhibitors reduce cardiovascular deaths, heart failure hospitalizations, hyperkalemia, and decline of renal function in patients with HFrEF.