
Pharmacists support acute pain management in patients on MOUD through education, optimizing therapy, guiding analgesia, and ensuring safe transitions of care.

Pharmacists support acute pain management in patients on MOUD through education, optimizing therapy, guiding analgesia, and ensuring safe transitions of care.

New studies reveal a modest, temporary increase in breast cancer risk linked to hormonal contraceptives, emphasizing the need for informed patient counseling.

Legislation aims to enhance health care access by allowing Medicare reimbursement for pharmacist services in rural areas, improving patient care and convenience.

Tools Like Robotic Dispensing Services, Pharmacogenomics, and Regulatory Changes Are Changing How Pharmacy Works

With Growing Patient Interest in Natural Remedies, Pharmacists Must Understand the Science, Safety, and Limitations of Essential Oils

Olezarsen effectively lowers triglycerides and enhances cardiovascular health, especially when combined with fibrates, benefiting pharmacists and patients alike.
New research reveals significant nonrespiratory complications in hospitalized children with influenza, highlighting the need for improved clinical awareness and antiviral treatment.
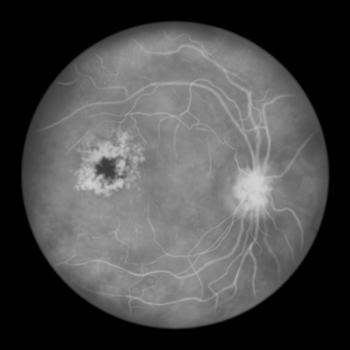
FDA approves an 8-mg aflibercept injection for macular edema following retinal vein occlusion (RVO), offering flexible dosing options for improved patient care.


Advanced IHC assays enhance HER2-low breast cancer detection, improving treatment eligibility for trastuzumab deruxtecan.
Particularly, younger women with chronic kidney disease (CKD) experienced the greatest survival disadvantage.

Counsel patients about effective remedies for pediatric colds, sore throats, and safe OTC options for adults with hypertension to alleviate symptoms.

Proactive Monitoring and Patient Education Are Key Roles for Pharmacists

Emergency medicine pharmacists discuss how they used a gamified, PowerPoint-based virtual escape room to improve disaster preparedness training, strengthen critical thinking, and highlight the essential role of pharmacists in emergency management.

Pharmacists Can Educate Patients About Symptoms of Menopause, Associated Chronic Conditions, and Management Options
CDC's recent statement raises concerns about vaccine safety and autism links, igniting debate among health experts and fueling vaccine hesitancy.

Dexcom Smart Basal optimizes basal insulin dosing for type 2 diabetes, enhancing management with personalized recommendations and a 15-day CGM sensor.

The findings from the BATURA clinical trial were presented at the 2025 ACAAI Annual Scientific Meeting.

A recent study reveals diverse long COVID trajectories, highlighting the need for further research to understand symptoms and recovery variability.

The biosimilars can be used to treat osteoporosis and cancer-related bone loss in certain populations.

New triglyceride-lowering therapy Olezarsen significantly reduces severe hypertriglyceridemia and acute pancreatitis risk, offering hope for patients.
The program’s legitimacy rests on transparency, audits, and ensuring benefits reach vulnerable patients.

The research further emphasizes the need for more attention on both chronic kidney disease (CKD) and mental health conditions.
New RSV vaccines for older adults enhance prevention efforts, yet awareness and uptake remain low. Pharmacists play a crucial role in improving vaccination rates.

Natural strategies and OTC options can help manage menopause symptoms, from hot flashes to mood changes, for a smoother transition.

The approval is based on results from the phase 3 DeLLphi-304 clinical trial.
FDA approves daratumumab and hyaluronidase for treating newly diagnosed light chain (AL) amyloidosis, enhancing survival rates and treatment efficacy.
The FDA accelerates approval of sevabertinib for advanced non–small cell lung cancer, showcasing promising efficacy and manageable safety in clinical trials.
