Top news of the day from across the health care landscape.
Research explores why patients with mutated KRAS genes in tumor cells are sensitive to cetuximab.
Minocycline topical foam, 4% is indicated for the treatment of inflammatory lesions of non-nodular moderate-to-severe acne vulgaris in adults and pediatric patients aged 9 years and older

Why is a healthy young woman suffering from hair loss?

Here are some ideas to help you celebrate the pharmacy profession all week long.
Four pharmaceutical distributors have agreed to settle federal litigation related to the ongoing opioid epidemic for a collective $260 million, less than a day before a federal trial was set to begin in Ohio.

Limited supply of chemotherapy agent that treats several pediatric cancers may lead to rationing of doses.

Ravulizumab-cwvz (Ultomiris, Alexion) is indicated to inhibit complement-mediated thrombotic microangiopathy in adult and pediatric patients with atypical hemolytic uremic syndrome.
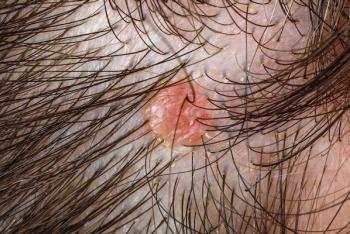
Three abstracts that evaluate the efficacy and safety of cantharidin (VP-102) were presented by Verrica Pharmaceuticals at the 39th Annual Fall Clinical Dermatology Conference in Las Vegas, Nevada.

One highlight of the American Society for Emergency Contraception (ASEC) EC Jamboree was the key clinical discussions centered on drug interactions between emergency contraception and other hormonal contraceptives, including ulipristal acetate.

The open-label Phase 2 trial looked to expand treatment for patients with newly diagnosed and persistent immune thrombocytopenia.

The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) agreed on a positive opinion for AbbVie’s upadacitinib (RINVOQ), according to the company.
Top news of the day from across the health care landscape.

After participating in a sleep-education program, 41% of participants who survived cancer had their insomnia successfully treated.

Top news of the week from Specialty Pharmacy Times.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

Pending results of the California Practice Standards and Jurisprudence Exam (CPJE) have been invalidated for 1400 applicants that recently took the test, due to a cheating issue.
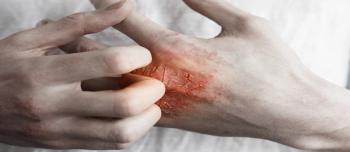
Abrocitinib showed improvements in skin clearance and itch relief in patients aged 12 years and older with moderate-to-severe atopic dermatitis.

A new Health Trends study released by Quest Diagnostics has highlighted many physicians' overconfidence in their ability to identify and treat substance misuse, as well as concerns that the drug gabapentin is increasingly being misused.

Glenmark Pharmaceuticals has received FDA approval for abiraterone acetate tablets USP, 250 mg, a generic of Janssen's Zytiga tablets, 250 mg.1

Which Halloween costumes are generating the most buzz this year?
Baloxavir marboxil (Xofluza, Genentech) is the first antiviral medication indicated for patients at high risk of developing influenza-related complications.

Although the demand for pharmacists is expected to increase in a variety of health care settings, the employment of pharmacists in this industry is projected to decline overall in the next 10 years.
Study suggests that blocking a signaling pathway that includes the inflammatory factor CXCL10-CXCR3 may prevent brain metastasis.
Top news of the day from across the health care landscape.
Nine widely-used medications have experienced substantial price growth since 2016, adding $5.1 billion to overall drug spending.

The full-day meeting will feature recognized experts sharing the latest clinical data in oncology, while exploring best practices and management strategies.

Kyowa Kirin’s istradefylline (Nourianz) is now available in the United States as adjunctive treatment to levodopa/carbidopa in adult patients with Parkinson’s disease (PD) that are experiencing “off” episodes.
The International Society of Oncology Pharmacy Practitioners global position on oncology biosimilar use covers regulation and evaluation, implementation, education, and monitoring.

Top news of the day from across the health care landscape.