
The FDA has granted approval of Rybelsus (semaglutide) oral tablets 7 mg or 14 mg to Novo Nordisk. The drug is the first oral glucagon-like peptide (GLP-1) receptor protein treatment for type 2 diabetes.

The FDA has granted approval of Rybelsus (semaglutide) oral tablets 7 mg or 14 mg to Novo Nordisk. The drug is the first oral glucagon-like peptide (GLP-1) receptor protein treatment for type 2 diabetes.

Specialty pharmacies are uniquely positioned to assist prescribers in managing patient care and offsetting administrative burden.

Obesity-associated cancer burden is shifting toward younger individuals, with more dramatic increases in some populations than others.
Top news of the day from across the health care landscape.
Top news of the day across the health care landscape.

Vitamin D helps the immune system stay balanced, much like a gymnist walking a balance beam.
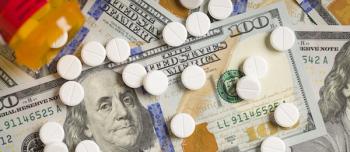
The new report found that widely used prescription drugs increased by an average of 4.2% in 2017, double the general inflation rate of 2.1%.






Founded in 2006, Project Health has delivered more than $127 million in free health care services to nearly 1.7 million Americans. The campaign offers a no-cost comprehensive health assessment that can detect early risks for chronic conditions such as diabetes, hypertension, and heart disease.
Transplanting organ from patients infected with hepatitis C could greatly expand the number of kidneys available and reduce wait times.

Jessica Langley, Executive Director of Education and Advocacy, National Healthcareer Association (NHA) discusses why pharmacies should be investing in their pharmacy technicians.

Top news of the day across the health care landscape.
New approach involves treating patients with a high dose factor VIII concentrates along with emicizumab, a modification of the standard immune tolerance induction.

Pharmacists for Healthier Lives (PfHL) announced their launch of a new campaign aimed at educating the public on the importance of immunizations to maintain a healthy population.

The FDA has approved Erleada (apalutamide) for the treatment of metastatic castration-sensitive prostate cancer (mCSPC), based on the results from the Phase 3 TITAN study.

A recent clinical study demonstrated positive long-term benefits of the early intervention combination treatment of metformin plus vildagliptin (Galvus, Norvartis), a dipeptidyl peptidase-4 [DPP-4] inhibitor, for type 2 diabetes (T2D).
Top news of the day from across the health care landscape.
Patients with metastatic colorectal cancer harboring certain non-V600 BRAF mutations may benefit from anti-EGFR treatment.

Researchers have found that methylnaltrexone, a peripherally acting µ-opioid receptor antagonist, may offer an effective solution to relieving patients' opioid-induced constipation with minimal adverse effects.

The FDA approval of pembrolizumab plus lenvatinib for this new indication is the first under a new international collaboration with Australia and Canada.
Two phase 3 studies showed that ofatumumab was superior to teriflunomide (Aubagio) in patients with relapsing forms of multiple sclerosis.

Research demonstrates a new direction in cell signaling and treatment for chronic inflammatory diseases such as psoriasis, asthma, and HIV.

The annual National Association of Specialty Pharmacy annual meeting shed light on the significant role these caregivers play in patient care.

The parties included in this agreement in principle represent some of the more than 2,600 lawsuits filed against Purdue Pharma concerning opioids, including the company’s OxyContin products.

What is causing the loss of blood pressure control?