The phase 2 study will evaluate the efficacy and safety of 2 different doses of MK-2060 in patients with ESRD receiving hemodialysis via an arteriovenous graft.

The phase 2 study will evaluate the efficacy and safety of 2 different doses of MK-2060 in patients with ESRD receiving hemodialysis via an arteriovenous graft.

The 4 academic centers will evaluate the role of telehealth in areas such as prevention, screening, diagnosis, treatment, and survivorship.
Analysis includes nearly 43 million individuals aged 13 year and older who received at least 1 dose in England between December 1, 2020, and December 15, 2021.

George Rafferty and Jason Dinger of AmerisourceBergen discuss the investment interests of the venture capital fund AB Health Ventures within the retail pharmacy space.
Survey data indicates that people experiencing homelessness had a lower annual incidence of COVID-19 despite having a higher burden of infectious diseases, suggesting opportunities for better integration of homelessness status in infectious disease reporting.

Precise, more potent therapy can offer reduced toxicity.
A systematic review of literature found that family history of psychiatric disorders is a risk factor associated with postpartum depression, adding to a contradictory body of research.

This month's generic product new features vilazadone tablets, leuprolide acetate injection, and oxcarbazepine tablets.

Evaluating nonprescription medications, including dietary supplements, can prevent adverse effects.
Results also show that no association with an increased frequency of myocarditis, venous thromboembolism, or worsening HF in these individuals.

High-level ethical decision making considers various ethical components and all stakeholders.

The mRNA-1273.222 vaccine candidate targets both the original SARS-CoV-2 strain in addition to the BA.4/BA.5 subvariants of the Omicron strain.

Danish analysis looks at the relationship between the therapy and cardiovascular adverse effects, particularly arrhythmias.
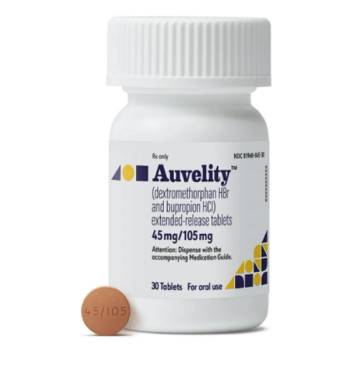
Auvelity is a combination of dextromethorphan and bupropion indicated for the treatment of major depressive disorder in adults.
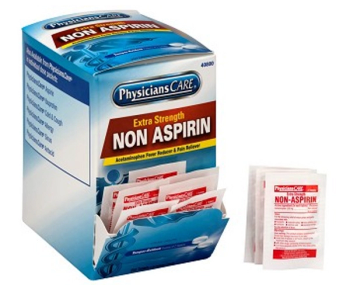
Acetaminophen helps to reduce fevers and treat headaches, pains, and menstrual cramps.

Women are 70% more likely to survive stage 1 breast cancer than stage 4, and new research shows that earlier screening could catch breast cancer earlier, increasing their survival rate.
Long-term follow up on the KEYNOTE-604 study suggests pembrolizumab plus etoposide/platinum is a superior first-line therapy for small cell lung cancer vs placebo.

In New York’s medical cannabis program, research showed that patients’ preference around potency differed among users, regardless of their health conditions.
The companies are prepared to deliver doses of the Omicron-adapted bivalent vaccine for September, pending authorization.
Less than half of fully vaccinated individuals in the United States have received a COVID-19 booster dose, with lower uptake rates among individuals with less education, lower socioeconomic status, and current enrollment in Medicaid.

Pharmacy Times will be covering the National Association of Chain Drug Stores (NACDS) Total Store Expo, taking place in Boston from August 27 to 29, 2022.

Irinotecan liposomal injection (Onivyde) used as second-line therapy for patients with small cell lung cancer did not meet the primary end point of overall survival compared to topotecan.

Jake Nichols, PharmD, CEO, president, and co-founder of Renovo Health, discusses the stigma around drug diversion and the best ways to address and fight that stigma.

Executives from CVS Health said they have multiple pathways in achieving a push to establish primary care services, provider enablement, and home health.

Investigators also report that the therapy could mimic the efficacy of olanzapine but cancelled out the medication-induced weight gain, improving symptoms and outcomes.
A P79S ORF2 variant demonstrates reduced RNA copy numbers released in the supernatant and impaired the production of infectious disease particles, investigators said.

Investigators report that while lateral flow tests do not detect the start of infectiousness well, they can identify individuals who will not infect others and can safely leave isolation.

Building a team to support best practices, medication safety, and staff education can reduce risks.
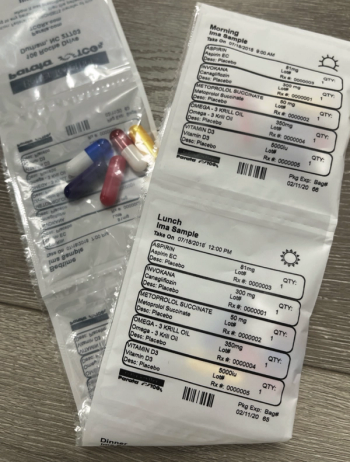
Dundee Pharmacy’s compliance packaging encourages patients to stay adherent to their medications.
Francesca Ceddia, MD, senior vice president of respiratory vaccines at Moderna, said the company is in early stages of development for a combined mRNA vaccine for influenza, COVID-19, and RSV.