Specifically, 3 new drugs for sickle cell disease are providing options for patients who are not responding to hydroxyurea.

Specifically, 3 new drugs for sickle cell disease are providing options for patients who are not responding to hydroxyurea.

In addition to improving working conditions for current employees, burnout must be addressed in order to recruit and retain new employees and alleviate understaffing.

Since 2014, 10 immunotherapies have been approved by the FDA, including 6 CAR T-cell therapies and 4 BiTE therapies.

Although state efforts can be impactful, Downs said enforcement is a major hurdle.
Spherix Global Insights estimates that SER-109 (Seres Therapeutics; Aimmune) will be able to challenge fecal microbiota, live-jslm in C. difficile treatment.

Phase 2B clinical trial results demonstrate that patients experienced improvement in total body and facial repigmentation.
Children in the LT-001 study treated after onset achieved or maintained additional milestones up to 7.5 years after 1-time intravenous infusion, Novartis says.

This month, we spoke with expert Adi Zuloff-Shani, PhD, CEO of Clearmind Medicine.

Rezafungin (Rezzayo; Cidara Therapeutics Inc, Melinta Therapeutics LLC) is the first new treatment option approved for individuals with candidemia and invasive candidiasis in more than a decade.

As the risk of respiratory depression is elevated with opioid use, the risk of complications from COVID-19 is increased in patients with opioid use disorder.

Among patients with lung cancer, approximately 20% have the earliest stage of disease but are still dying of metastatic lung cancer within about 5 years.

Drugs such as pembrolizumab, sacituzumab govitecan, and datopotamab deruxtecan all have growing bodies of evidence illustrating which patients with cancer are most likely to benefit and in which settings.
Different guidelines have notable differences between equivalency dosing of proton pump inhibitors (PPI), resulting in confusion and a lack of confidence between medical professionals recommending one PPI over another.

Products include oral suspension for gout and tablets that prevent low carnitine levels in the blood.

Research has even shown that cancer diagnosis can be significantly improved using AI, with real-time data updates, personalized attention, and ultimately better results at lower costs.
The test did not reach the World Health Organization’s minimum performance requirement, which is 80% sensitivity for SARS-CoV-2, in the study population.

Prevention, role of advanced machine learning systems, safety should be top of mind for next 12 months and beyond

Despite tensions that can occur, building relationships with health systems and finding opportunities to collaborate is crucial to improve cancer care.

Expert Cmdr. Jennifer Lind, PharmD, MPH, MBA, discussed how her work in the CDC and the USPHS is a unique path for pharmacists interested in public health.

Children who died from fatal poisoning were often at home and in the presence of an adult.
In an additional post-hoc analysis, guselkumab demonstrated improvements at week 48, according to Janssen Pharmaceutical.

The treatment shows positive results in metastasis-free survival for men with non-metastatic hormone-sensitive prostate cancer, Pfizer and Astellas announce.

Shiela Plasencia, director of practice support at the Community Oncology Alliance, discussed COA’s new tool to help practices prepare for the Enhancing Oncology Model.
3D biofabrication models reflect increasing global awareness of the ethics of preclinical studies and an increased need for determining therapeutic efficacy differences.

Nick Ferreyros, managing director of policy, advocacy, and communications at the Community Oncology Alliance, discussed the Alliance’s efforts surrounding PBMs and 340B reform.
Retifanlimab-dlwr (Zynyz; Incyte) was granted FDA accelerated approval for metastatic or recurrent locally advanced Merkel cell carcinoma.

There is no single thing that can prevent all cancer risk, but there are 6 pillars of lifestyle that people can adopt to best prevent the disease.

Kandy Dunnigan, AAS, CPhT-Adv, a drug diversion analyst at Novant Health, discusses her role in the pharmacy.
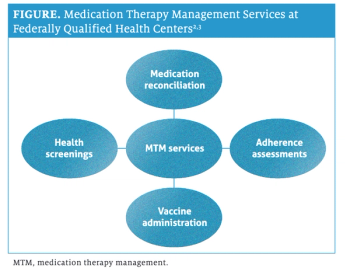
Services include adherence assessments, health screenings, medication reconciliation, vaccine administration.

Strong interprofessional team helps ensure safe transitions and minimize adverse events, medication errors