
Truvada (emtricitabine/tenofovir disoproxil fumarate) was approved for prophylaxis to reduce the risk of HIV-1 infection in adults.

Truvada (emtricitabine/tenofovir disoproxil fumarate) was approved for prophylaxis to reduce the risk of HIV-1 infection in adults.

There have been significant increases in the cost of antiretroviral therapies (ART) for individual therapies and in the cost of therapy initiation over the past several years.

Making birth control pills available OTC would increase women's access to them and reduce the rate of unintended pregnancy, but issues such as reimbursement for pharmacist services still need to be worked out, the group argues.

New recommendations from the task force say that all Americans aged 15 to 64 years should get an HIV test regardless of their risk for contracting the virus.
In a session at the 2012 Academy of Managed Care Pharmacy Educational Conference, Aimee Tharaldson, PharmD, identified the top specialty drugs in the pipeline and highlighted the most recent therapeutic trends within each medication class.

The latest advances in HIV treatment and care include new drug therapies, a new at-home test, and updates guidelines for antiretroviral therapy for pregnant women.



Patients taking Truvada may still significantly reduce their risk of HIV infection even when they are not 100% adherent to their drug regimens, a new study revealed.

The Johnson & Johnson Patient Assistance Foundation, Inc (JJPAF) announced that it has started to use a new common patient assistance program application for the HIV medicines it offers, streamlining for patients in the United States the process of applying for HIV medicines from multiple patient assistance programs.


The generic marketplace remains dynamic and complicated, as patent expirations,interest in biosimilars, and international competition spur major changes.



Stribild, previously known as the Quad, has been approved by the FDA to treat HIV-1 infection in adults who are starting HIV treatment for the first time.
The FDA has expanded the indication of Merck's Isentress (raltegravir) to include pediatric patients.


The FDA approved an expanded indication for Isentress (raltegravir) to include the treatment of HIV-1 infection in children 2 years and older.
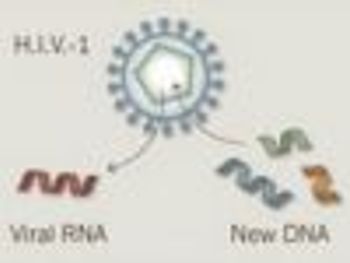
A new research and licensing partnership between the University of Pennsylvania and Novartis aims to develop more effective treatments for cancer by giving patients a disabled form of HIV.

Findings of a new study suggest women infected with HIV do not need more frequent Pap tests.

Treatment with antiretrovirals is now recommended for all adults with HIV infection, regardless of their CD4 count.
The initiative will provide greater insight into how the patient-pharmacist relationship can help improve retention in care, adherence and viral suppression outcomes.
The FDA approved Truvada for preexposure prophylaxis, making it the first agent ever to be approved for HIV prevention in uninfected adults.


The FDA today approved OraQuick In-Home HIV Test, the first over-the-counter HIV test that gives users results without having to send samples off to a lab.