
Prescription-only technology with insurance reimbursement helps patients and prescribers better manage diabetes.

Prescription-only technology with insurance reimbursement helps patients and prescribers better manage diabetes.
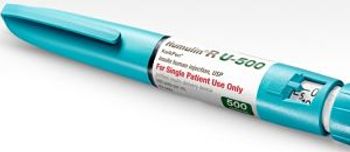
The FDA today approved Eli Lilly's Humulin R U-500 insulin human injection (KwikPen) for patients with type 1 and type 2 diabetes who need more than 200 units of insulin per day.

Metformin decreased inflammation and scarring observed in more common pancreatic cancers.

Diabetes associated with a 60% increased risk of dementia for both men and women.

Diabetes associated with a 60% increased risk of dementia for both men and women.

Because diabetes is among the most common conditions for which pharmacies will dispense medications, a number of pharmacy technicians seek to better understand the disease.

Patients with type 2 diabetes have about a 60% greater risk for developing dementia than their non-diabetic counterparts.

Many patients with diabetes have high levels of mental illness, multiple comorbidities, and unmet needs.

There are some specific questions that pharmacist should ask when counseling type 2 diabetics.
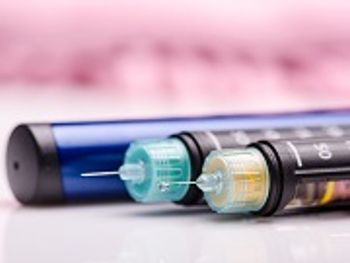
Caregivers will be able to monitor glucose data and administer insulin right from the pump.

Caregivers will be able to monitor glucose data and administer insulin right from the pump.

Children ages 2 to 17 years will now be allowed to use the Animas Vibe insulin pump and continuous glucose monitoring system for diabetes management.
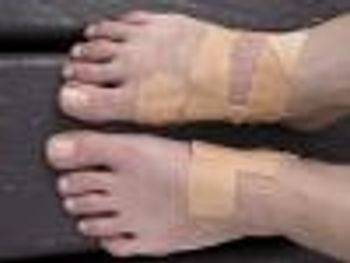
Device should be used in conjunction with standard diabetic ulcer care.
Disappointing sales were blamed on prior authorization, the FDA label restrictions, and the need for spirometry before doctors could write prescriptions.

Patients with certain diabetic foot ulcers now have a new treatment option.
Pharmacists are powerful advocates for insulin adherence in patients with diabetes.
A middle-aged man experienced complete remission of type 2 diabetes after cure of chronic hepatitis C virus.
New drug application filed for fixed-ratio combination of insulin glargine and lixisenatide.

Sanofi has submitted a new drug application to the FDA for a fixed-ratio combination of insulin glargine and lixisenatide.

Patients with cerebral palsy are often faced with secondary health conditions such as diabetes, asthma, and hypertension.
Protein linked to diabetic enteropathy.

Arguments persist regarding which diabetes patients should be prescribed blood pressure-lowering treatments.

High blood pressure is an established risk factor for cardiovascular events in patients with diabetes.

Basaglar improves glycemic control among adult and pediatric patients with type 1 diabetes and among adults with type 2 diabetes.

The FDA has approved insulin glargine injection, a long-acting human insulin analog to improve glycemic control among adult and pediatric patients with type 1 diabetes and adults with type 2 diabetes.