
The guidance said relaxing certain measures for vaccinated individuals may help improve COVID-19 vaccine acceptance and uptake.

The guidance said relaxing certain measures for vaccinated individuals may help improve COVID-19 vaccine acceptance and uptake.

The investigators urged continued safety measures, such as social distancing and school closures, in order to prevent more COVID-19 variant-related deaths and hospitalizations.
In a recent interview with Pharmacy Times, Council Powell, PharmD, region director with CVS, said pharmacists and technicians are hard at work administering COVID-19 vaccines around the country.
Pharmacy Times® interviewed Farah Towfic, PharmD, MBA, the director and CEO of operations at US Pharmacopeia, on the FDA’s approval of the Pfizer and BioNTech COVID-19 vaccine’s updated cold storage requirements.
The first vaccine dose alone offered strong protection against COVID-19, with efficacy that did not decline over the 3 months between doses.

ACIP issued an interim recommendation to the Janssen COVID-19 vaccine for use in individuals aged ≥18 years for the prevention of COVID-19.

President Joseph Biden announced a partnership between Merck and Johnson & Johnson, in which 2 of Merck’s facilities will begin producing the Johnson & Johnson COVID-19 vaccine.
Throughout the COVID-19 pandemic, patients hospitalized with the disease have had more to face than just the fight against COVID-19, as co-infection with drug-resistant pathogens has been shown to also contribute to rates of mortality and readmission.

Amesh Adalja, MD, FIDSA, FACP, FACEP, discussed the distribution prospects for the Johnson & Johnson COVID-19 vaccine in light of it being possible to store it at standard refrigeration temperatures for up to 3 months.
A recent study found that a combination of robust vaccination programs and strict physical distancing rules may be effective in the prevention of future COVID-19 infection surges.

Although there have been many challenges and concerns during the pandemic, experts said there are also lessons to be learned and implemented once the pandemic is over.
The CDC recommends that patients with cancer and COVID-19 have a conversation with their health care provider or care team to discuss the individual level of risk based on the patient’s current condition, treatment, and the level of transmission within their community.

APhA commended President Biden’s signing of the executive order in a press release, noting that adequate drug supply would be critical to ensuring pharmacists’ ability to provide care to patients during the COVID-19 pandemic.

Amesh Adalja, MD, FIDSA, FACP, FACEP, a senior scholar at the Johns Hopkins Center for Health Security, discusses how Johnson & Johnson’s COVID-19 vaccine provides effective support in prevention of severe disease and hospitalization.

It has taken both time and effort to address the equitable allocation of COVID-19 vaccinations, and in communities such as Prince George’s County in Maryland, significant effort has been made to address barriers to access.

In the LinkedIn Live session, “The Road Toward Vaccine Equity Webinar,” Walgreens highlighted their efforts toward helping fill the important gaps in care that address the unique concerns of minority communities.

As a majority of advanced pharmacy practice experiences need to include direct patient care, immunizing provides students an opportunity to provide this care along with consultation around the COVID-19 vaccine and adverse effects.

Approximately 2 million doses of Johnson & Johnson's single-dose COVID-19 vaccine are ready to ship and supply is expected to sharply increase, with up to 20 million doses available by the end of March.
In voting in favor of the recommendation, 22 committee members unanimously agreed that the benefits of the Johnson & Johnson COVID-19 vaccine outweigh its risks.

The flexibility could allow for easier storage and increased accessibility of the vaccine.
Pharmacy Times® interviewed Amesh Adalja, MD, FIDSA, FACP, FACEP, a senior scholar at the Johns Hopkins Center for Health Security, on how the proposed EUA for Johnson & Johnson’s single-dose vaccine may impact the COVID-19 vaccine distribution and administration process.
Pharmacy Times® interviewed Kenneth Thorpe, PhD, the former deputy assistant secretary at the HHS during the Clinton administration, on how pandemic preparedness lessons from COVID-19 may help to tackle the antimicrobial resistance health crisis.

Study findings illustrate the need for better communication between health care providers and patients experiencing concerns during the pandemic.
The vaccine has been shown to induce mucosal immunity in the nasal cavity, which may be effective in blocking transmission of SARS-CoV-2.
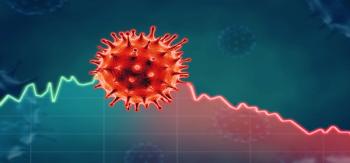
The current surge peaked on January 11, 2021, but took just 5 days to decrease by 10%, compared to 22 days following the first peak in April 2020.