The percentage dropped to only about 25% among people at a low genetic risk for the disease.

The percentage dropped to only about 25% among people at a low genetic risk for the disease.

The incidence of colorectal cancer in adults between the ages of 40 and 49 years has increased by nearly 15% since 2000.

The implementation of multi-gene panel testing may be a necessary part of the standard of care for all colorectal cancer patients.
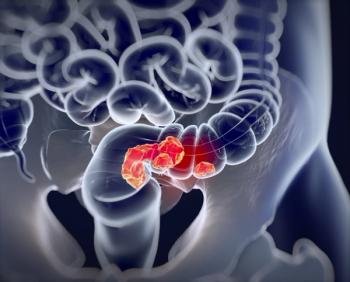
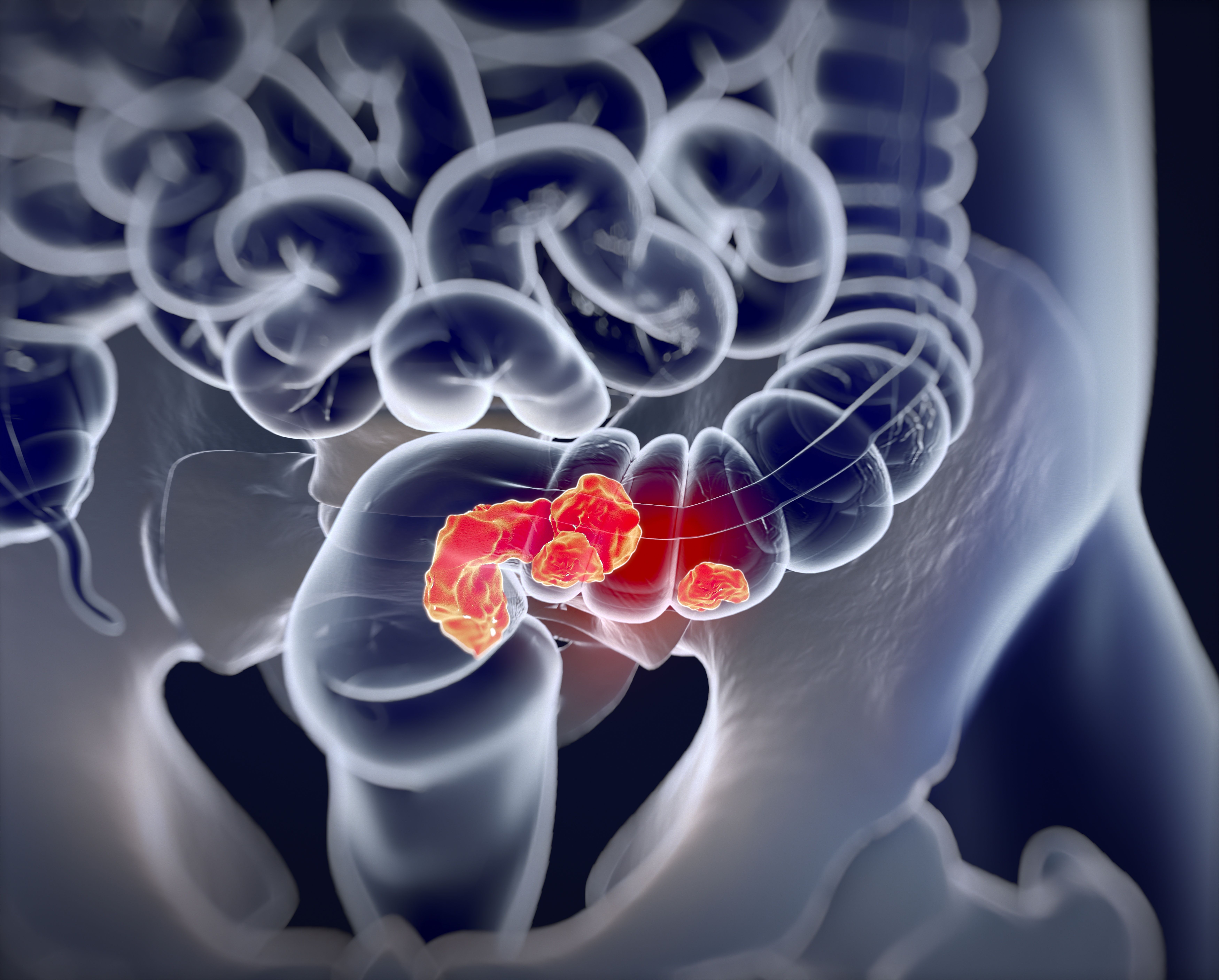
In June 2020, the FDA granted a fast track designation to fruquintinib for patients with mCRC previously treated with other therapies.
Individuals with an average risk of colorectal cancer said they would prefer a stool-based screening test for colorectal cancer over colonoscopy.

The study also analyzed the relationship between ultra-processed food and drink products with 2 other cancer types.

A new study published in The American Journal of Clinical Nutrition found that for colorectal cancer survivors, maintaining a stable body weight may hide a loss of muscle and the development of fatty deposits in their muscles, which resulted in a 40% higher risk of premature death.

The findings suggest pre-diagnosis aspirin use might help reduce CRC mortality in the overall population by limiting metastatic spread of colorectal tumors before diagnosis.

If approved, the biosimilar will be indicated for use in metastatic colorectal cancer, nonsquamous non–small cell lung cancer, recurrent glioblastoma, metastatic renal cell carcinoma, and metastatic cervical cancer.

Pharmacists should communicate the universal message that colorectal cancer screenings save lives.
A new combination therapy cleanses the colon before this important procedure that screens for colorectal cancer, the third-leading cause of cancer-related deaths in the United States.
The United States Preventive Services Task Force updates recommends that colorectal cancer screening begin 5 years earlier, at age 45 instead of age 50.

The COVID-19 pandemic has caused many routine colorectal cancer screenings to be delayed.

A patient has Lynch syndrome, a hereditary condition that puts young people at high risk for colon cancer.

By using a non-invasive screening model, care providers can help catch and treat more cases of colorectal cancer, a new study suggests.
Leung added that this is the first study to show the potential beneficial effects of ACE inhibitors and ARBs on colorectal cancer development, based on a large group of patients who were colorectal cancer-free at the beginning of the study.

According to a press release, this is the first single-agent, anti-PD-1 therapy approved in the first-line environment for this patient population.
Investigators found that less than a quarter of study participants with colorectal cancer were taking their medications as prescribed, suggesting that many could benefit from improved adherence.
The results of the new study were presented during the virtual scientific program of the 2020 American Society of Clinical Oncology (ASCO) Annual Meeting.
FDA has granted Fast Track designation to Cardiff Oncology’s onvansertib, an oral and highly-selective Polo-like Kinase 1 (PLK1) inhibitor, for the second-line treatment of patients with KRAS-mutated metastatic colorectal cancer.

New treatments with biological agents and vaccines are creating a pathway for patients with metastatic CRC.
Pharmacists are a rich resource, not only in the management of colorectal cancer, but in every aspect of health care.

The FDA has approved encorafenib in combination with cetuximab for the treatment of adult patients with metastatic colorectal cancer with a BRAF V600E mutation after prior therapy.

Moderate physical activity may improve progression-free survival and reduce treatment-related adverse effects in patients undergoing chemotherapy for advanced colorectal cancer.

Cancers of the lung, breast, prostate, and colon are most common in adults aged 85 years and older.