Inappropriately prescribed medications can increase the risk of serious adverse effects and increase the cost of care.

Inappropriately prescribed medications can increase the risk of serious adverse effects and increase the cost of care.

Black women experience longer waiting periods before treatment is initiated than White women following a breast cancer diagnosis, as well as a prolonged treatment duration compared with White women.

The current acute treatments for migraines have shown efficacy and safety, however, approximately one-third of people do not respond, cannot use these options due to contraindications, or report dissatisfaction in therapy.
Angiotensin receptor II blockers are often prescribed to control hypertension or for heart failure, kidney failure, or following a heart attack.
The United States Preventive Services Task Force updates recommends that colorectal cancer screening begin 5 years earlier, at age 45 instead of age 50.
Study to evaluate paclitaxel plus encequidar with dostarlimab +/- carboplatin in patients with breast cancer.
Researchers have identified the role of a protein involved in the progression of hepatitis C virus (HCV) infection that has demonstrated a potential new avenue for treatment.
Simple sugar found in breast milk and dietary supplements may promote myelin repair in patients with multiple sclerosis.

FDA lift clinical hold placed on the phase 1 PSMA-101-001 study of the chimeric antigen receptor T-cell therapy P-PSMA-101 in patients with metastatic castration-resistant prostate cancer.
FDA approves the cobas EGFR Mutation Test v2 as a companion diagnostic for EGFR TKIs in the treatment of EGFR-mutated non–small cell lung cancer.
The FDA granted priority review to a supplemental biologics license application for cemiplimab-rwlc for the frontline treatment of patients with locally advanced or metastatic non–small cell lung cancer.
Currently, genetic counselors apply their molecular and clinical expertise in diverse roles, many delivered via telehealth.

Approximately 30% of women gain weight following chemotherapy for breast cancer, gut bacteria may be why.

The FDA granted priority review to a supplemental biologics license application for trastuzumab deruxtecan in patients with HER2-positive gastric or gastroesophageal junction adenocarcinoma.
FDA approves FoundationOne Liquid CDx as a companion diagnostic for alpelisib in advanced or metastatic breast cancer, rucaparib in advanced ovarian cancer, and alectinib in a specific type of metastatic non–small cell lung cancer.
Both intravenous immunoglobulin and subcutaneous immunoglobulin are effective in treating myasthenia gravis.
An axillary lymph node dissection, which is the surgical removal of 10 to 20 lymph nodes from the breast, is a procedure conducted in up to 25% of women with breast cancer to disable symptoms of arm swelling.
Lipid-lowering medication may be an effective adjuvant cancer therapy.
Approximately half of the people with multiple sclerosis (MS) have the HLA-DR15 gene variant, which is responsible for up to 60% of genetic risk.

During the COVID-19 pandemic, pharmacists have been forced to face challenges to their practice that have required not only adaptation and flexibility, but also moments of empathy and vulnerability.

The FDA has approved the FoundationOne CDx (F1CDx) comprehensive genomic test as a companion diagnostic for larotrectinib (Vitrakvi) to identify patients with NTRK1/2/3 gene fusions across all solid tumors.
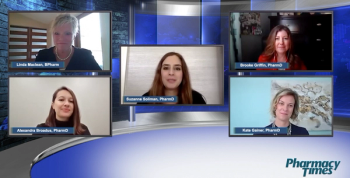
On Women Pharmacist Day on October 12, a panel discussion webcast consisting of leading women in pharmacy discussed the importance of recognizing the impact of women on the pharmacy landscape and the history of women in the field.

Ubrogepant (Ubrelvy, Allergan) was approved for the treatment of acute migraines with or without aura in adults after showing a significantly greater reduction in headache pain compared with placebo.

Pharmacy Times®, in conjunction with Parata Systems, recognized the 2020 winners of the Next-Generation Pharmacist awards at the 11th annual virtual awards gala.
VX-765, a novel multiple sclerosis drug, works by inhibiting a component of inflammasomes that promotes harmful inflammation.

Allison Burns, Pharm.D., RPh, honored as the 2020 Next-Generation Pharmacist®.
Cholesterol-lowering statins have been found to potentially reduce the risk of cancer via a pathway that is unrelated to its impact on cholesterol, according to a recent study.
The FDA granted fast track designation to the investigational CD20-directed monoclonal antibody ublituximab in combination with the PI3K-delta inhibitor umbralisib for the treatment of chronic lymphocytic leukemia.

Patients with current or former smoking status may be at an increased risk of severe illness due to COVID-19.

The FDA granted osimertinib (Tagrisso) priority review designation for the adjuvant treatment of patients with early-stage EGFR-mutated non–small cell lung cancer following complete tumor resection with curative intent.