Novel process may identify cancer metastasis at an earlier stage when it may be easier to treat.

Novel process may identify cancer metastasis at an earlier stage when it may be easier to treat.

Cancers of the lung, breast, prostate, and colon are most common in adults aged 85 years and older.
Research highlights the importance of regular blood testing to impove earlier detection of multiple myeloma.

Top news of the day from across the health care landscape.

Doug Long, vice president of Industry Relations at IQVIA, discusses rebate reform surrounding Medicare Part D and Part B in an interview with Specialty Pharmacy Times.

A phase 3 study evaluated the safety and efficacy of oral paclitaxel versus intravenous paclitaxel monotherapy in patients with metastatic breast cancer.
Aspirin therapy found to improve liver function test results and survival after transarterial embolization for hepatocellular carcinoma.

Top news of the day from across the health care landscape.

Study recommends similar clinical thresholds for diagnosing and managing giant cell arteritis for patients of different ethnicities.

Upadacitinib (Rinvoq, AbbVie) is indicated for adults with moderate-to-severe rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate.

A phase 4 head-to-head trial compared ixekizumab (Taltz) with guselkumab (Tremfya) in patients with moderate-to-severe plaque psoriasis.
Top news of the day from across the health care landscape.

Top news of the week from Specialty Pharmacy Times.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

The approval of fedratinib (Inrebic, Celgene) provides another treatment option for patients with myelofibrosis, a rare bone marrow disorder.

Officials with the FDA have granted approval to entrectinib (Rozlytrek, Genentech) for 2 different cancer indications.

Top news of the day from across the health care landscape.

HIV-related immunosuppression may contribute to an elevated risk of cancer-related morbidity in elderly patients with the disease.

If approved, acalabrutinib (Calquence, AstraZeneca) will offer a chemotherapy-free treatment option for adults with chronic lymphocytic leukemia.

An analysis of a quality improvement project at an oncology specialty pharmacy showed that pharmacist interventions can reduce financial toxicity for patients.

Top news of the day from across the health care landscape.

High body mass index is associated with an increased risk of chronic diseases such as type 2 diabetes, cancer, musculoskeletal, and respiratory conditions.

Artificial intelligence able to recognize patterns in gene sequences and molecular data from breast cancer, which oncologists are now evaluating in clinical trials.
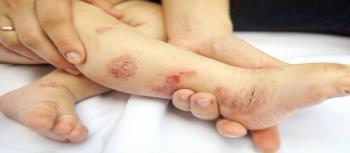
A phase 3 trial evaluated dupilumab (Dupixent, Regeneron and Sanofi) in children from 6 to 11 years of age with severe atopic dermatitis.

Top news of the day from across the health care landscape.
The number of first-degree relatives affected by blood cancer and age of diagnosis may contribute to the likelihood of a family member’s risk of being diagnosed with the same disease.

Investigators believe that urine sampling can be used to diagnose and treat bladder cancer.

Top news of the day from across the health care landscape.
In an ideal world, how would you hypothesize that technology might be able to solve all of your problems?

Doug Long, MBA, vice president of industry relations at IQVIA, discusses how vertical integration will affect health care delivery.