Company releases data at the American Society of Clinical Oncology Annual Meeting 2022 showing that the drug is well-tolerated in patients with solid tumors.

Company releases data at the American Society of Clinical Oncology Annual Meeting 2022 showing that the drug is well-tolerated in patients with solid tumors.

There are a huge number of medical facilities in Ukraine that run international clinical trials, with the FDA noting that more than 250 drugs and devices were undergoing clinical research in Ukraine.
The new study analyzed KSHV’s latent-lytic switch, a process in which the virus exits its dormancy state to replicate in the host cell.

Pegfilgrastim-pbbk is the third oncology biosimilar developed by Amneal to gain FDA approval this year.
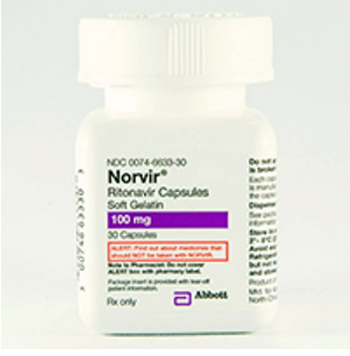
Ritonavir (Norvir) is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.
Health systems must have a flexible workflow in place to allow the use of non-preferred agents to accommodate payer demands and reduce financial toxicity.

Nivolumab (Opdivo) with fluoropyrimidine- and platinum-containing chemotherapy and nivolumab plus ipilimumab (Yervoy) approved as first-line treatments for unresectable advanced or metastatic esophageal squamous cell carcinoma.

Further sensitivity analyses to control for family alcohol consumption, family history, liver disease, and smoking, finds no convincing evidence that these factors affected the results.
The approval is supported by data from a global phase 3 trial assessing the use of the drug for individuals with previously untreated IDH1-mutated acute myeloid leukemia.

Karen Fancher, PharmD, BCOP, a member of the Patient Advisory Panel at the Hematology/Oncology Pharmacy Association (HOPA), explains how her experience as a patient with cancer and an oncology pharmacy specialist informs her work on HOPA’s Patient Advisory Panel.

Byron Yoshino, PharmD, CEO of Pharmacare Hawaii, discusses the future of the home infusion space within the United States in the coming years.

Dong Xu, PhD, MS, curators' distinguished professor at the University of Missouri College of Engineering, discusses how artificial intelligence is being used to develop new drug therapies for medical treatments targeting cancers and other diseases.

Byron Yoshino, PharmD, CEO of Pharmacare Hawaii, discusses the rapidly growing home infusion space within Pharmacare Hawaii’s network of pharmacies.
Xofigo is indicated for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases, and no known visceral metastatic disease.

Study results also show a 50% increase in the precancerous condition, Barrett esophagus, between 2012 and 2019, in the same population.

Specialty pharmacy is deeply engaged in providing treatment for conditions that are at high risk for mental health challenges.

Latest results of the phase 3 IKEMA trial demonstrate the longest median progression-free survival on a proteasome inhibitor backbone in this population, Sanofi says.
Independent committee recommends the phase 3 clinical trial continues to assess other primary and secondary endpoints for the treatment of unresectable or metastatic urothelial carcinoma.

Nakia Eldridge, PharmD, MBA, director of health care quality, safety, and information at US Pharmacopeia (USP), discusses points of note regarding USP’s work in setting and supporting public quality standards.
:Simeprevir (Olysio) is an NS3/4A protease inhibitor indicated for the treatment of chronic hepatitis C infection as a component of a combination antiviral treatment regimen.

The FDA previously placed a clinical hold on lenacapavir in borosilicate vials in all clinical studies because of emerging concerns about the compatibility of vials made of borosilicate glass.

Aklilu Tedla, vice president of strategy and business development at Cardinal Health, discusses the cell and gene therapies in the pipeline for approval by the FDA.

Aklilu Tedla, vice president of strategy and business development at Cardinal Health, discusses the future of pharmacies as more cell and gene therapy products become available to patients.

Repotrectinib awarded breakthrough therapy designation for patients with ROS1-positive metastatic non–small cell lung cancer previously treated with a ROS1 tyrosine kinase inhibitor and who were not previously administered platinum-based chemotherapy.
The SKYSCRAPER-01 trial evaluating the drug plus Tecentriq demonstrates that it did not meet its co-primary endpoint of progression-free survival.

They should consider many factors and use clear guidance on predicting phenotypes from genotypes to intervene optimally based on specific drug-gene interactions.

Doug Long, vice president, Industry Relations, IQVIA, discusses notable trends reshaping specialty pharmaceutical channels and the impact of these developments on the role of the pharmacist in the health care team.
Vosevi is a fixed-dose combination for the treatment of hepatitis C virus.

Joel Wayment, vice president of operations for 3PL Services at Cardinal Health, discusses how the pharmaceutical supply chain currently manages the transport of CAR T products to sites of administration.

Ray Tancredi, divisional vice president of Specialty Pharmacy, Development & Brand Rx/Vaccine Purchasing at Walgreens, discusses blockbuster drugs in the oncology space that have been approved this year or may be hitting the market soon.